Cognitive Outcomes after DEXmedetomidine sedation in cardiac surgery: CODEX randomised controlled trial protocol
- PMID: 33849856
- PMCID: PMC8051371
- DOI: 10.1136/bmjopen-2020-046851
Cognitive Outcomes after DEXmedetomidine sedation in cardiac surgery: CODEX randomised controlled trial protocol
Abstract
Introduction: Older patients undergoing cardiac surgery carry the highest risk for developing major postoperative neurocognitive disorder (postoperative NCD or P-NCD) with up to 25% incidence 3 months after surgery. P-NCD is associated with significant morbidity, mortality, loss of independence, premature retirement and increased healthcare costs. This multicentre randomised trial is investigating the efficacy of postoperative dexmedetomidine sedation in reducing the incidence of major P-NCD after cardiac surgery compared with standard protocols. CODEX will be the largest interventional trial with major P-NCD as the primary outcome.
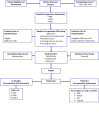
Methods and analysis: CODEX is recruiting patients ≥60 years old, undergoing elective cardiac surgery and without pre-existing major cognitive dysfunction or dementia. Eligible participants are randomised to receive postoperative dexmedetomidine or standard institutional sedation protocols in the intensive care unit. Baseline preoperative cognitive function is assessed with the computer-based Cogstate Brief Battery. The primary outcome, major P-NCD, 3 months after surgery is defined as a decrease in cognitive function ≥1.96 SD below age-matched, non-operative controls. Secondary outcomes include delirium, major P-NCD at 6/12 months, depressive symptoms, mild P-NCD and quality of surgical recovery at 3/6/12 months. The specific diagnostic criteria used in this protocol are consistent with the recommendations for clinical assessment and management of NCD from the Nomenclature Consensus Working Group on perioperative cognitive changes. Intention-to-treat analysis will compare major P-NCD at 3 months between study groups.
Ethics and dissemination: CODEX was approved by Sunnybrook Health Sciences Centre Research Ethics Board (REB) (Project ID 1743). This will be the first multicentre, randomised controlled trial to assess the efficacy of a pharmacological intervention to reduce the incidence of major P-NCD after cardiac surgery in patients ≥60 years old. Dissemination of the study results will include briefings of key findings and interpretation, conference presentations and peer-reviewed publications.
Trial registration number: NCT04289142.
Keywords: adult anaesthesia; anaesthesia in cardiology; cardiac surgery; clinical trials; delirium & cognitive disorders; neurology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
