Dissociating Perceptual Awareness and Postperceptual Processing: The P300 Is Not a Reliable Marker of Somatosensory Target Detection
- PMID: 33849946
- PMCID: PMC8260252
- DOI: 10.1523/JNEUROSCI.2950-20.2021
Dissociating Perceptual Awareness and Postperceptual Processing: The P300 Is Not a Reliable Marker of Somatosensory Target Detection
Abstract
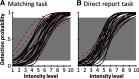
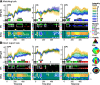
A central challenge in the study of conscious perception lies in dissociating the neural correlates of perceptual awareness from those reflecting its precursors and consequences. No-report paradigms have been instrumental in this endeavor, demonstrating that the event-related potential P300, recorded from the human scalp, reflects reports rather than awareness. However, these paradigms cannot probe the degree to which stimuli are consciously processed from trial to trial and, thus, leave open the possibility that the P300 is a genuine correlate of conscious access enabling reports. Here, instead of removing report requirements, we took the opposite approach and equated postperceptual task demands across conscious and unconscious trials by orthogonalizing target detection and overt reports in a somatosensory detection task. We used Bayesian model selection to track the transformation from physical to perceptual processing stages in the EEG data of 24 male and female participants and show that the early P50 component scaled with physical stimulus intensity, whereas the N140 component was the first correlate of target detection. The late P300 component was elicited for both perceived and unperceived stimuli and was not substantially modulated by target detection. This was in stark contrast to a control experiment using a classical direct report task, which replicated the P50 and N140 effects but additionally showed a strong effect of target detection in the P300 time range. Our results demonstrate the task dependence of the P300 in the somatosensory modality and show that late cortical potentials dissociate from perceptual awareness even when stimuli are always reported.SIGNIFICANCE STATEMENT The time it takes for sensory information to enter our conscious experience can be an indicator of the neural processing stages that lead to perceptual awareness. However, because many cognitive processes routinely correlate with perception, isolating those signals that uniquely reflect perceptual awareness is not a trivial task. Here, we show that late electroencephalography signals cease to correlate with somatosensory awareness when common task confounds are controlled. Importantly, by balancing report requirements instead of abolishing them, we show that the lack of late effects cannot be explained by a lack of conscious access. Instead, we propose that conscious access occurs earlier, at ∼150 ms, supporting the view that early activity in sensory cortices is a neural correlate of conscious perception.
Keywords: Bayesian model selection; P300; electroencephalography; perceptual awareness; somatosensory; target detection.
Copyright © 2021 the authors.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
