Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice
- PMID: 33850049
- PMCID: PMC8092378
- DOI: 10.1073/pnas.2022489118
Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice
Abstract
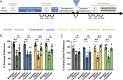
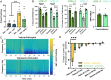
Depression is a widespread and devastating mental illness and the search for rapid-acting antidepressants remains critical. There is now exciting evidence that the psychedelic compound psilocybin produces not only powerful alterations of consciousness, but also rapid and persistent antidepressant effects. How psilocybin exerts its therapeutic actions is not known, but it is widely presumed that these actions require altered consciousness, which is known to be dependent on serotonin 2A receptor (5-HT2AR) activation. This hypothesis has never been tested, however. We therefore asked whether psilocybin would exert antidepressant-like responses in mice and, if so, whether these responses required 5-HT2AR activation. Using chronically stressed male mice, we observed that a single injection of psilocybin reversed anhedonic responses assessed with the sucrose preference and female urine preference tests. The antianhedonic response to psilocybin was accompanied by a strengthening of excitatory synapses in the hippocampus-a characteristic of traditional and fast-acting antidepressants. Neither behavioral nor electrophysiological responses to psilocybin were prevented by pretreatment with the 5-HT2A/2C antagonist ketanserin, despite positive evidence of ketanserin's efficacy. We conclude that psilocybin's mechanism of antidepressant action can be studied in animal models and suggest that altered perception may not be required for its antidepressant effects. We further suggest that a 5-HT2AR-independent restoration of synaptic strength in cortico-mesolimbic reward circuits may contribute to its antidepressant action. The possibility of combining psychedelic compounds and a 5-HT2AR antagonist offers a potential means to increase their acceptance and clinical utility and should be studied in human depression.
Keywords: depression; hallucination; hallucinogen; psychedelic; serotonin.
Conflict of interest statement
Competing interest statement: The University of Maryland Baltimore has filed a provisional patent, in which S.M.T. is listed as an inventor on the use of psychedelics combined with 5-HT2R antagonists to treat psychiatric disease.
Figures

References
-
- Nutt D., Erritzoe D., Carhart-Harris R., Psychedelic psychiatry’s brave new world. Cell 181, 24–28 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

