Characterization of combined linagliptin and Y2R agonist treatment in diet-induced obese mice
- PMID: 33850212
- PMCID: PMC8044192
- DOI: 10.1038/s41598-021-87539-7
Characterization of combined linagliptin and Y2R agonist treatment in diet-induced obese mice
Abstract
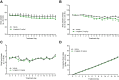
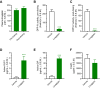
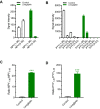
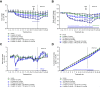
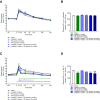
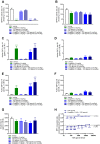
Dipeptidyl peptidase IV (DPP-IV) inhibitors improve glycemic control by prolonging the action of glucagon-like peptide-1 (GLP-1). In contrast to GLP-1 analogues, DPP-IV inhibitors are weight-neutral. DPP-IV cleavage of PYY and NPY gives rise to PYY3-36 and NPY3-36 which exert potent anorectic action by stimulating Y2 receptor (Y2R) function. This invites the possibility that DPP-IV inhibitors could be weight-neutral by preventing conversion of PYY/NPY to Y2R-selective peptide agonists. We therefore investigated whether co-administration of an Y2R-selective agonist could unmask potential weight lowering effects of the DDP-IV inhibitor linagliptin. Male diet-induced obese (DIO) mice received once daily subcutaneous treatment with linagliptin (3 mg/kg), a Y2R-selective PYY3-36 analogue (3 or 30 nmol/kg) or combination therapy for 14 days. While linagliptin promoted marginal weight loss without influencing food intake, the PYY3-36 analogue induced significant weight loss and transient suppression of food intake. Both compounds significantly improved oral glucose tolerance. Because combination treatment did not further improve weight loss and glucose tolerance in DIO mice, this suggests that potential negative modulatory effects of DPP-IV inhibitors on endogenous Y2R peptide agonist activity is likely insufficient to influence weight homeostasis. Weight-neutrality of DPP-IV inhibitors may therefore not be explained by counter-regulatory effects on PYY/NPY responses.
Conflict of interest statement
HHH, RVG, and LKL are employed by Gubra; NV and JJ are owners of Gubra; TB-P, PH and TK are employed by Boehringer Ingelheim Pharma; HT is employed by PXBioVisioN. This study was supported by Boehringer Ingelheim Pharma.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

