Advances in immunotherapy for hepatocellular carcinoma
- PMID: 33850328
- PMCID: PMC8042636
- DOI: 10.1038/s41575-021-00438-0
Advances in immunotherapy for hepatocellular carcinoma
Abstract
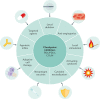
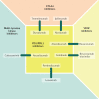
Hepatocellular carcinoma (HCC) is a prevalent disease with a progression that is modulated by the immune system. Systemic therapy is used in the advanced stage and until 2017 consisted only of antiangiogenic tyrosine kinase inhibitors (TKIs). Immunotherapy with checkpoint inhibitors has shown strong anti-tumour activity in a subset of patients and the combination of the anti-PDL1 antibody atezolizumab and the VEGF-neutralizing antibody bevacizumab has or will soon become the standard of care as a first-line therapy for HCC, whereas the anti-PD1 agents nivolumab and pembrolizumab are used after TKIs in several regions. Other immune strategies such as adoptive T-cell transfer, vaccination or virotherapy have not yet demonstrated consistent clinical activity. Major unmet challenges in HCC checkpoint immunotherapy are the discovery and validation of predictive biomarkers, advancing treatment to earlier stages of the disease, applying the treatment to patients with liver dysfunction and the discovery of more effective combinatorial or sequential approaches. Combinations with other systemic or local treatments are perceived as the most promising opportunities in HCC and some are already under evaluation in large-scale clinical trials. This Review provides up-to-date information on the best use of currently available immunotherapies in HCC and the therapeutic strategies under development.
© 2021. Springer Nature Limited.
Conflict of interest statement
I.M. reports advisory roles with Roche-Genentech, Bristol-Myers Squibb, CYTOMX, Incyte, MedImmune, Tusk, F-Star, Genmab, Molecular Partners, Alligator, Bioncotech, MSD, Merck Serono, Boehringer Ingelheim, Astra Zeneca, Numab, Catalym and Bayer, and research funding from Roche, BMS, Alligator and Bioncotech. B.S. reports consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical and Terumo and speaker fees from Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical, Terumo BMS and Sirtex Medical. P.S. and S.H.-S. report no competing interests.
Figures


References
-
- WHO. Liver. http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

