The impact of phthalate on reproductive function in women with endometriosis
- PMID: 33850448
- PMCID: PMC8022091
- DOI: 10.1002/rmb2.12364
The impact of phthalate on reproductive function in women with endometriosis
Abstract
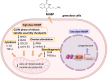
Background: Endometriosis is a common gynecological condition in which stromal or glandular epithelium is implanted in extrauterine locations. Endometriosis causes detrimental effects on the granulosa cells, and phthalate interferes with the biological and reproductive function of endometrial cells at a molecular level.
Methods: This article retrospectively reviewed the studies on phthalate exposure and its relationship with endometriosis. A literature search was performed for scientific articles using the keywords "phthalate and endometriosis," "endometriosis and granulosa cells," "phthalate and granulosa cells," and "phthalates and endometrial cells."
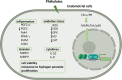
Results: Endometriosis can affect cytokine production, steroidogenesis, cell cycle progression, expression of estrogen receptor-α (ER-α)/progesterone receptor (PR), and cause endoplasmic reticulum stress, senescence, apoptosis, autophagy, and oxidative stress in the granulosa cells. Mono-n-butyl phthalate (MnBP) alters the expression of cytokines, cell cycle-associated genes, ovarian stimulation, steroidogenesis, and progesterone production. Several in vitro studies have demonstrated that phthalate caused inflammation, invasion, change in cytokines, increased oxidative stress, viability, resistance to hydrogen peroxide, and proliferation of endometrial cells.
Conclusion: This might provide new insights about the impact of phthalate on the pathogenesis of endometriosis and its consequences on the ovarian function.
Keywords: endometrial cells; endometriosis; granulosa cells; phthalate; reproductive function.
© 2021 The Authors. Reproductive Medicine and Biology published by John Wiley & Sons Australia, Ltd on behalf of Japan Society for Reproductive Medicine.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any study with human or animal participants that have been performed by any of the authors.
Figures

Similar articles
-
Exposure to Mono-n-Butyl Phthalate in Women with Endometriosis and Its Association with the Biological Effects on Human Granulosa Cells.Int J Mol Sci. 2020 Mar 5;21(5):1794. doi: 10.3390/ijms21051794. Int J Mol Sci. 2020. PMID: 32151056 Free PMC article.
-
Phthalate exposure and risk of ovarian dysfunction in endometriosis: human and animal data.Front Cell Dev Biol. 2023 Jul 25;11:1154923. doi: 10.3389/fcell.2023.1154923. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37560165 Free PMC article.
-
Phthalates and risk of endometriosis.Environ Res. 2013 Oct;126:91-7. doi: 10.1016/j.envres.2013.07.003. Epub 2013 Jul 25. Environ Res. 2013. PMID: 23890968 Free PMC article.
-
Association between the Exposure to Phthalates and the Risk of Endometriosis: An Updated Review.Biomedicines. 2024 Aug 22;12(8):1932. doi: 10.3390/biomedicines12081932. Biomedicines. 2024. PMID: 39200395 Free PMC article. Review.
-
Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact.J Steroid Biochem Mol Biol. 2016 Jan;155(Pt A):35-46. doi: 10.1016/j.jsbmb.2015.07.023. Epub 2015 Sep 25. J Steroid Biochem Mol Biol. 2016. PMID: 26407755 Review.
Cited by
-
Morin hydrate treatment minimizes Di(2-ethylhexyl) phthalate-induced uterine fibrosis, oxidative stress, and apoptosis in mice.Mol Biol Rep. 2025 Mar 13;52(1):308. doi: 10.1007/s11033-025-10423-4. Mol Biol Rep. 2025. PMID: 40080243
-
Cross-sectional associations between phthalates, phenols, and parabens with metabolic syndrome risk during early-to-mid adolescence among a cohort of Mexican youth.Environ Res. 2023 Nov 1;236(Pt 1):116706. doi: 10.1016/j.envres.2023.116706. Epub 2023 Jul 19. Environ Res. 2023. PMID: 37474091 Free PMC article.
References
-
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002;955:89‐100; discussion 118, 396–406. - PubMed
-
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. - PubMed
-
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244‐1256. - PubMed
-
- Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1‐15. - PubMed
-
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789‐1799. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
