Paeoniflorin protects PC12 cells from oxygen-glucose deprivation/reoxygenation-induced injury via activating JAK2/STAT3 signaling
- PMID: 33850544
- PMCID: PMC8027733
- DOI: 10.3892/etm.2021.10004
Paeoniflorin protects PC12 cells from oxygen-glucose deprivation/reoxygenation-induced injury via activating JAK2/STAT3 signaling
Abstract
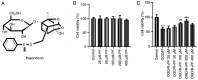
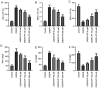
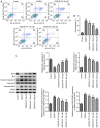
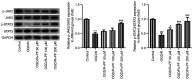
Ischemic stroke is the most common type of stroke, and it has become a major health issue as it is characterized by high mortality and morbidity rates. Paeoniflorin (PF) is a natural compound and the main active ingredient of Radix Paeoniae. The aim of the present study was to investigate the role of PF in oxygen-glucose deprivation/reoxygenation (OGD/R)-induced injury of PC12 cells and its association with the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway. An in vitro model of OGD/R injury was established in PC12 cells. Subsequently, Cell Counting Kit-8 assay and ELISA were used to evaluate cell viability and the secretion of inflammatory factors, respectively, in PC12 cells subjected to OGD/R and treated with PF. The levels of oxidative stress indicators and inflammatory factors were measured using corresponding commercial kits. In addition, the apoptosis rate of PC12 cells subjected to OGD/R and treated with PF was determined by flow cytometry, and the expression of apoptosis-related proteins was analyzed by western blotting. Additionally, the expression levels of JAK2/STAT3 pathway-related proteins were also evaluated. The cell viability, levels of oxidative stress, inflammation and apoptosis were also measured in OGD/R-induced PC12 cell injury models following co-treatment of cells with PF and FLLL32, a specific inhibitor of JAK2/STAT3 signaling. Cell viability was reduced, while oxidative stress and inflammation were increased after OGD/R-induced injury. However, the treatment of cells with PF significantly enhanced cell viability, and alleviated oxidative stress, inflammation and apoptosis of OGD/R-treated PC12 cells. Furthermore, PF activated the JAK2/STAT3 signaling pathway. Following FLLL32 intervention, the effects of PF on oxidative stress, inflammation and apoptosis of OGD/R-treated PC12 cells were reversed. In conclusion, the findings of the present study suggested that PF may protect PC12 cells from OGD/R-induced injury via activating the JAK2/STAT3 signaling pathway, thus providing novel insight into the mechanism through which PF may alleviate ischemic stroke and indicating a potential strategy for ischemic stroke treatment.
Keywords: apoptosis; inflammation; ischemic stroke; oxygen-glucose deprivation/reoxygenation; paeoniflorin.
Copyright: © Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Preclinical Evidence of Paeoniflorin Effectiveness for the Management of Cerebral Ischemia/Reperfusion Injury: A Systematic Review and Meta-Analysis.Front Pharmacol. 2022 Apr 8;13:827770. doi: 10.3389/fphar.2022.827770. eCollection 2022. Front Pharmacol. 2022. PMID: 35462929 Free PMC article.
-
Protective effects of the knockdown of lncRNA AK139328 against oxygen glucose deprivation/reoxygenation-induced injury in PC12 cells.Mol Med Rep. 2021 Sep;24(3):621. doi: 10.3892/mmr.2021.12260. Epub 2021 Jul 2. Mol Med Rep. 2021. PMID: 34212979 Free PMC article.
-
SH2B1 protects against OGD/R‑induced apoptosis in PC12 cells via activation of the JAK2/STAT3 signaling pathway.Mol Med Rep. 2018 Sep;18(3):2613-2620. doi: 10.3892/mmr.2018.9265. Epub 2018 Jul 9. Mol Med Rep. 2018. PMID: 30015896 Free PMC article.
-
Astragaloside IV Alleviates Cerebral Ischemia-Reperfusion Injury by Activating the Janus Kinase 2 and Signal Transducer and Activator of Transcription 3 Signaling Pathway.Pharmacology. 2020;105(3-4):181-189. doi: 10.1159/000503361. Epub 2019 Dec 11. Pharmacology. 2020. PMID: 31825924
-
Effects of JAK2-STAT3 signaling after cerebral insults.JAKSTAT. 2014 Jun 12;3(2):e29510. doi: 10.4161/jkst.29510. eCollection 2014. JAKSTAT. 2014. PMID: 25105066 Free PMC article. Review.
Cited by
-
Preclinical Evidence of Paeoniflorin Effectiveness for the Management of Cerebral Ischemia/Reperfusion Injury: A Systematic Review and Meta-Analysis.Front Pharmacol. 2022 Apr 8;13:827770. doi: 10.3389/fphar.2022.827770. eCollection 2022. Front Pharmacol. 2022. PMID: 35462929 Free PMC article.
-
Design, synthesis, and evaluation of the novel ozagrel-paeonol codrug with antiplatelet aggregation activities as a potent anti-stroke therapeutic agent.Front Pharmacol. 2024 Mar 19;15:1362857. doi: 10.3389/fphar.2024.1362857. eCollection 2024. Front Pharmacol. 2024. PMID: 38567356 Free PMC article.
-
Cholinesterase Inhibitory Activity of Paeoniflorin: Molecular Dynamics Simulation, and In Vitro Mechanistic Investigation.Biochem Res Int. 2024 Dec 19;2024:9192496. doi: 10.1155/bri/9192496. eCollection 2024. Biochem Res Int. 2024. PMID: 39735856 Free PMC article.
-
Plant-derived polyphenolic compounds for managing schizophrenia: mechanisms and therapeutic potential.Front Pharmacol. 2025 Jun 19;16:1605027. doi: 10.3389/fphar.2025.1605027. eCollection 2025. Front Pharmacol. 2025. PMID: 40612741 Free PMC article. Review.
-
Paeoniflorin mitigates insulin-like growth factor 1-induced lipogenesis and inflammation in human sebocytes by inhibiting the PI3K/Akt/FoxO1 and JAK2/STAT3 signaling pathways.Nat Prod Bioprospect. 2024 Oct 1;14(1):56. doi: 10.1007/s13659-024-00478-4. Nat Prod Bioprospect. 2024. PMID: 39349732 Free PMC article.
References
-
- Martini ML, Neifert SN, Lara-Reyna JJ, Shuman WH, Ladner TR, Hardigan TH, Fifi JT, Mocco J, Yaeger KA. Trials in thrombectomy for acute ischemic stroke: Describing the state of clinical research in the field. Clin Neurol Neurosurg. 2021;200(106360) doi: 10.1016/j.clineuro.2020.106360. - DOI - PubMed
-
- Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
