lncRNA LOC102724169 plus cisplatin exhibit the synergistic anti-tumor effect in ovarian cancer with chronic stress
- PMID: 33850634
- PMCID: PMC8010577
- DOI: 10.1016/j.omtn.2021.03.001
lncRNA LOC102724169 plus cisplatin exhibit the synergistic anti-tumor effect in ovarian cancer with chronic stress
Abstract
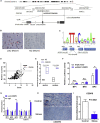
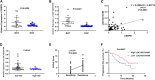
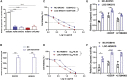
Chronic stress has been proven to accelerate the development and progression of ovarian cancer, but the underlying molecular mechanisms have not been fully elucidated. In a combination survey of ovarian cancer with chronic stress (OCCS) mouse models and high-throughput sequencing, a key lncRNA named LOC102724169 on chromosome 6q27 has been identified, which functions as a dominant tumor suppressor in OCCS. Transcriptionally regulated by CCAAT enhancer binding protein (CEBP) beta (CEBPB), LOC102724169 shows low expression and correlates with poor progression-free survival (PFS) in OCCS patients. LOC102724169 is an instructive molecular inhibitor of malignancy of ovarian cancer cells, which is necessary to improve the curative effect of cisplatin therapy on ovarian cancer. This function stems from the inactivation of molecules in phosphatidylinositol 3-kinase (PI3K)/AKT signaling, repressing MYB expression and retaining the responsiveness of cancer cells to cisplatin. These findings provide a mechanistic understanding of the synergistic anti-tumor purpose of LOC102724169 as a bona fide tumor suppressor, enhancing the therapeutic effect of cisplatin. The new regulatory model of "lncRNA-MYB" provides new perspectives for LOC102724169 as a chronic stress-related molecule and also provides mechanistic insight into exploring the cancer-promoting mechanism of MYB in OCCS, which may be a promising therapeutic strategy for ovarian cancer.
Keywords: chronic stress; ciaplatin resistance; lncRNA LOC102724169; ovarian cancer; tumor suppressor.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
A novel PI3K/mTOR dual inhibitor, CMG002, overcomes the chemoresistance in ovarian cancer.Gynecol Oncol. 2019 Apr;153(1):135-148. doi: 10.1016/j.ygyno.2019.01.012. Epub 2019 Jan 25. Gynecol Oncol. 2019. PMID: 30686552
-
4-Acetylantroquinonol B suppresses autophagic flux and improves cisplatin sensitivity in highly aggressive epithelial cancer through the PI3K/Akt/mTOR/p70S6K signaling pathway.Toxicol Appl Pharmacol. 2017 Jun 15;325:48-60. doi: 10.1016/j.taap.2017.04.003. Epub 2017 Apr 10. Toxicol Appl Pharmacol. 2017. PMID: 28408137
-
MicroRNA-17 promotes normal ovarian cancer cells to cancer stem cells development via suppression of the LKB1-p53-p21/WAF1 pathway.Tumour Biol. 2015 Mar;36(3):1881-93. doi: 10.1007/s13277-014-2790-3. Epub 2014 Dec 16. Tumour Biol. 2015. PMID: 25510663
-
Systematic analysis reveals a lncRNA-mRNA co-expression network associated with platinum resistance in high-grade serous ovarian cancer.Invest New Drugs. 2018 Apr;36(2):187-194. doi: 10.1007/s10637-017-0523-3. Epub 2017 Oct 30. Invest New Drugs. 2018. PMID: 29082457
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
Cited by
-
Intricate crosstalk between MYB and noncoding RNAs in cancer.Cancer Cell Int. 2021 Dec 7;21(1):653. doi: 10.1186/s12935-021-02362-4. Cancer Cell Int. 2021. PMID: 34876130 Free PMC article. Review.
-
Identification of a Novel Immune-Related lncRNA CTD-2288O8.1 Regulating Cisplatin Resistance in Ovarian Cancer Based on Integrated Analysis.Front Genet. 2022 Feb 14;13:814291. doi: 10.3389/fgene.2022.814291. eCollection 2022. Front Genet. 2022. PMID: 35237300 Free PMC article.
-
Emerging role of MYB transcription factors in cancer drug resistance.Cancer Drug Resist. 2024 Apr 30;7:15. doi: 10.20517/cdr.2023.158. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38835346 Free PMC article. Review.
-
Latest Update on lncRNA in Epithelial Ovarian Cancer-A Scoping Review.Cells. 2025 Apr 7;14(7):555. doi: 10.3390/cells14070555. Cells. 2025. PMID: 40214508 Free PMC article.
-
Long non-coding RNAs in cancer: multifaceted roles and potential targets for immunotherapy.Mol Cell Biochem. 2024 Dec;479(12):3229-3254. doi: 10.1007/s11010-024-04933-1. Epub 2024 Feb 28. Mol Cell Biochem. 2024. PMID: 38413478 Review.
References
-
- Jayson G.C., Kohn E.C., Kitchener H.C., Ledermann J.A. Ovarian cancer. Lancet. 2014;384:1376–1388. - PubMed
-
- Thaker P.H., Han L.Y., Kamat A.A., Arevalo J.M., Takahashi R., Lu C., Jennings N.B., Armaiz-Pena G., Bankson J.A., Ravoori M. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12:939–944. - PubMed
-
- Idahl A., Hermansson A., Lalos A. Social support and ovarian cancer incidence—A Swedish prospective population-based study. Gynecol. Oncol. 2018;149:324–328. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

