Towards a new WHO classification of renal cell tumor: what the clinician needs to know-a narrative review
- PMID: 33850785
- PMCID: PMC8039604
- DOI: 10.21037/tau-20-1150
Towards a new WHO classification of renal cell tumor: what the clinician needs to know-a narrative review
Abstract
In 1952, renal cell carcinomas had been divided into 2 categories-clear cell or granular cell-depending upon their cytoplasmic staining characteristics. In the following years, the inventory of renal epithelial tumors has expanded by the addition of tumors named by their architectural pattern (i.e., papillary RCC, tubulocystic RCC), anatomic location (i.e., collecting duct carcinoma, renal medullary carcinoma), associated diseases (i.e., acquired cystic disease-associated RCCs). With the extensive application of molecular diagnostic techniques, it becomes possible to detect genetic distinctions between various types of renal neoplasm and discover new entities, otherwise misdiagnosed or diagnosed as unclassified RCC. Some tumors such as ALK rearrangement-associated RCC, MiT family translocation renal carcinomas, SDH-deficient renal cancer or FH-deficient RCC, are defined by their molecular characteristics. The most recent World Health Organization (WHO) classification of renal neoplasms account for more than 50 entities and provisional entities. New entities might be included in the upcoming WHO classification. The aim of this review is to summarise and discuss the newly acquired data and evidence on the clinical, pathological, molecular features and on the prognosis of new RCC entities, which will hopefully increase the awareness and the acceptance of these entities among clinicians and improve prognostication for individual patients.
Keywords: Renal cell carcinoma; Von Hippel-Lindau gene (VHL); anaplastic lymphoma kinase (ALK); classification; clear cell RCC; emerging entities; fumarate hydratase (FH); molecular pathology; non-clear cells RCC; succinate dehydrogenase (SDH).
2021 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1150). The series “Update on Molecular Classification and Individualized Treatments of Genitourinary Tumors” was commissioned by the editorial office without any funding or sponsorship. AC, LC, MS, ALB and RM served as the unpaid Guest Editors of the series. LC serves as an unpaid editorial board member of Translational Andrology and Urology from Dec 2018 to Nov 2022. The authors have no other conflicts of interest to declare.
Figures


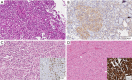
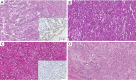
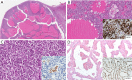
References
-
- Herbut PA. Urologic Pathology. Philadelphia, PA: Lea and Febiger; 1952.
-
- Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumors of the urinary system and male genital organs. Lyon, France: IARC; 2016. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
