Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset
- PMID: 33851143
- PMCID: PMC8030737
- DOI: 10.1016/j.medj.2021.04.003
Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset
Abstract
Background: RNA vaccines against coronavirus disease 2019 (COVID-19) have demonstrated ∼95% efficacy in phase III clinical trials. Although complete vaccination consisted of 2 doses, the onset of protection for both licensed RNA vaccines was observed as early as 12 days after a single dose. The adaptive immune response that coincides with this onset of protection could represent the necessary elements of immunity against COVID-19.
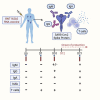
Methods: Serological and T cell analysis was performed in a cohort of 20 healthcare workers after receiving the first dose of the Pfizer/BioNTech BNT162b2 vaccine. The primary endpoint was the adaptive immune responses detectable at days 7 and 10 after dosing.
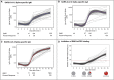
Findings: Spike-specific T cells and binding antibodies were detectable 10 days after the first dose of the vaccine, in contrast to receptor-blocking and severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) neutralizing antibodies, which were mostly undetectable at this early time point.
Conclusions: Our findings suggest that early T cell and binding antibody responses, rather than either receptor-blocking or virus neutralizing activity, induced early protection against COVID-19.
Funding: The study was funded by a generous donation from The Hour Glass to support COVID-19 research.
Keywords: COVID-19; RNA vaccine; T cells; binding antibodies; neutralizing antibodies; vaccine efficacy.
© 2021 The Author(s).
Conflict of interest statement
Duke-NUS Medical School is in partnership with Arcturus Therapeutics to develop a self-replicating RNA vaccine against COVID-19, with E.E.O. as the principal investigator. No monetary or personal benefits are derived from this partnership.
Figures

References
-
- Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020 doi: 10.1101/2020.12.09.20245175. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

