This is a preprint.
Nanobody Repertoires for Exposing Vulnerabilities of SARS-CoV-2
- PMID: 33851164
- PMCID: PMC8043454
- DOI: 10.1101/2021.04.08.438911
Nanobody Repertoires for Exposing Vulnerabilities of SARS-CoV-2
Update in
-
Highly synergistic combinations of nanobodies that target SARS-CoV-2 and are resistant to escape.Elife. 2021 Dec 7;10:e73027. doi: 10.7554/eLife.73027. Elife. 2021. PMID: 34874007 Free PMC article.
Abstract
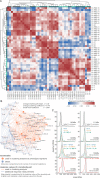
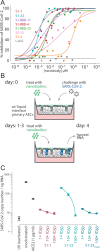
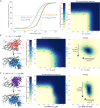
Despite the great promise of vaccines, the COVID-19 pandemic is ongoing and future serious outbreaks are highly likely, so that multi-pronged containment strategies will be required for many years. Nanobodies are the smallest naturally occurring single domain antigen binding proteins identified to date, possessing numerous properties advantageous to their production and use. We present a large repertoire of high affinity nanobodies against SARS-CoV-2 Spike protein with excellent kinetic and viral neutralization properties, which can be strongly enhanced with oligomerization. This repertoire samples the epitope landscape of the Spike ectodomain inside and outside the receptor binding domain, recognizing a multitude of distinct epitopes and revealing multiple neutralization targets of pseudoviruses and authentic SARS-CoV-2, including in primary human airway epithelial cells. Combinatorial nanobody mixtures show highly synergistic activities, and are resistant to mutational escape and emerging viral variants of concern. These nanobodies establish an exceptional resource for superior COVID-19 prophylactics and therapeutics.
Figures




References
-
- Bard G.V. (2007). Spelling-error tolerant, order-independent pass-phrases via the Damerau–Levenshtein string-edit distance metric. In Proceedings of the Fifth Australasian Symposium on ACSW Frontiers: 2007, Ballarat, Australia, January 30 - February 2, 2007 Conferences in Research and Practice in Information Technology (Darlinghurst, Australia: Australian Computer Society, Inc.), pp. 117–124.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
