This is a preprint.
Structural basis for broad sarbecovirus neutralization by a human monoclonal antibody
- PMID: 33851169
- PMCID: PMC8043460
- DOI: 10.1101/2021.04.07.438818
Structural basis for broad sarbecovirus neutralization by a human monoclonal antibody
Update in
-
Broad sarbecovirus neutralization by a human monoclonal antibody.Nature. 2021 Sep;597(7874):103-108. doi: 10.1038/s41586-021-03817-4. Epub 2021 Jul 19. Nature. 2021. PMID: 34280951 Free PMC article.
Abstract
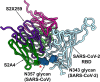
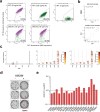
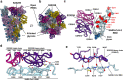
The recent emergence of SARS-CoV-2 variants of concern (VOC) and the recurrent spillovers of coronaviruses in the human population highlight the need for broadly neutralizing antibodies that are not affected by the ongoing antigenic drift and that can prevent or treat future zoonotic infections. Here, we describe a human monoclonal antibody (mAb), designated S2X259, recognizing a highly conserved cryptic receptor-binding domain (RBD) epitope and cross-reacting with spikes from all sarbecovirus clades. S2X259 broadly neutralizes spike-mediated entry of SARS-CoV-2 including the B.1.1.7, B.1.351, P.1 and B.1.427/B.1.429 VOC, as well as a wide spectrum of human and zoonotic sarbecoviruses through inhibition of ACE2 binding to the RBD. Furthermore, deep-mutational scanning and in vitro escape selection experiments demonstrate that S2X259 possesses a remarkably high barrier to the emergence of resistance mutants. We show that prophylactic administration of S2X259 protects Syrian hamsters against challenges with the prototypic SARS-CoV-2 and the B.1.351 variant, suggesting this mAb is a promising candidate for the prevention and treatment of emergent VOC and zoonotic infections. Our data unveil a key antigenic site targeted by broadly-neutralizing antibodies and will guide the design of pan-sarbecovirus vaccines.
Figures



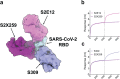






References
-
- Xianding Deng et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxiv: 10.1101/2021.03.07.21252647 (2021). - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
