Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies
- PMID: 33851875
- PMCID: PMC8054487
- DOI: 10.1080/14760584.2021.1903879
Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies
Abstract
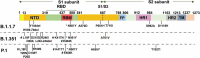
Introduction: As the global severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic continues to spread, several variants have emerged. Variants B.1.1.7 and B.1.351 have attracted significant attention owing to their widespread transmission and possible immune evasion. A total of 19 SARS-CoV-2 vaccines based on original strains have entered clinical studies, including nine vaccines that have obtained emergency use or conditional marketing authorizations. However, newly emerging variants may affect their protective efficacy. Decreased efficacy of the Novartis, Johnson & Johnson, and AstraZeneca vaccines against B.1.351 has been reported. The spread of variants creates a tremendous challenge for the prevention and control of the SARS-CoV-2 pandemic via vaccination. Several response strategies, including accelerating massive rollouts of current vaccines, increasing vaccine immunogenicity by increasing vaccination doses, and accelerating next-generation vaccines against variants, have been suggested.
Areas covered: SARS-CoV-2 vaccine efficacy against variants and response strategies for emerging variants.
Expert opinion: Current SARS-CoV-2 vaccines authorized for emergency use or under clinical trials have shown certain advantages in providing adequate protection against new variants. We analyzed the effects of reported variants on neutralizing antibodies and the protective efficacy of different vaccines and propose strategies for applying current vaccines against variants and developing next-generation vaccines.
Keywords: SARS-cov-2; neutralization; protective efficacy; vaccine; variant.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous