Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months
- PMID: 33852032
- PMCID: PMC8044656
- DOI: 10.1007/s00134-021-06388-0
Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months
Abstract
Purpose: Clinical characteristics and management of COVID-19 patients have evolved during the pandemic, potentially changing their outcomes. We analyzed the associations of changes in mortality rates with clinical profiles and respiratory support strategies in COVID-19 critically ill patients.
Methods: A multicenter cohort of RT-PCR-confirmed COVID-19 patients admitted at 126 Brazilian intensive care units between February 27th and October 28th, 2020. Assessing temporal changes in deaths, we identified distinct time periods. We evaluated the association of characteristics and respiratory support strategies with 60-day in-hospital mortality using random-effects multivariable Cox regression with inverse probability weighting.
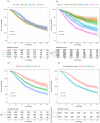
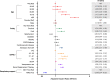
Results: Among the 13,301 confirmed-COVID-19 patients, 60-day in-hospital mortality was 13%. Across four time periods identified, younger patients were progressively more common, non-invasive respiratory support was increasingly used, and the 60-day in-hospital mortality decreased in the last two periods. 4188 patients received advanced respiratory support (non-invasive or invasive), from which 42% underwent only invasive mechanical ventilation, 37% only non-invasive respiratory support and 21% failed non-invasive support and were intubated. After adjusting for organ dysfunction scores and premorbid conditions, we found that younger age, absence of frailty and the use of non-invasive respiratory support (NIRS) as first support strategy were independently associated with improved survival (hazard ratio for NIRS first [95% confidence interval], 0.59 [0.54-0.65], p < 0.001).
Conclusion: Age and mortality rates have declined over the first 8 months of the pandemic. The use of NIRS as the first respiratory support measure was associated with survival, but causal inference is limited by the observational nature of our data.
Keywords: Coronavirus; In-hospital mortality; Non-invasive ventilation; Respiratory support.
Conflict of interest statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MS and JIFS are founders and equity shareholders of Epimed Solutions®, which commercializes the Epimed Monitor System®, a cloud-based software for ICU management and benchmarking. The other authors declare that they have no conflict of interest.
Figures

Comment in
-
If not now, when? A clinical perspective on the unprecedented challenges facing ICUs during the COVID-19 pandemic.Intensive Care Med. 2021 May;47(5):588-590. doi: 10.1007/s00134-021-06404-3. Epub 2021 May 11. Intensive Care Med. 2021. PMID: 33974107 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

