Atrioventricular block after fingolimod resumption: a consequence of sphingosine-1-phosphate axis alteration due to COVID-19?
- PMID: 33852086
- PMCID: PMC8045443
- DOI: 10.1007/s00415-021-10556-z
Atrioventricular block after fingolimod resumption: a consequence of sphingosine-1-phosphate axis alteration due to COVID-19?
Abstract
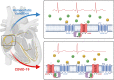
During the COVID-19 pandemic, concerns raised regarding the use of immunosuppressants in multiple sclerosis, even if current data do not support an increased risk of infection. Although fingolimod can be temporarily suspended during COVID-19, the benefit-risk balance of suspension can be challenging. Till now, no adverse events have been described after the resumption of fingolimod, following a previous discontinuation. We report the occurrence of atrioventricular block following fingolimod restart. Fingolimod acts on sphingosine-1-phosphate-axis, a pathway that is altered with COVID-19 and hypoxic conditions. Herein we discuss how these metabolic changes may have influenced fingolimod pharmacology leading to a cardiac event.
Keywords: Atrioventricular block; COVID-19; Fingolimod; Sphingosine 1-phosphate.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
M. Orrico, S. Gelibter and A. Nozzolillo have no conflicts of interest to report. F. Sangalli has received speaker’s honoraria and travel support form Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and TEVA. P. Preziosa received speaker honoraria from Biogen Idec, Novartis, Merck Serono and ExceMED. L. Moiola has received speaker’s honoraria from the following companies: Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and TEVA. M. Filippi is Editor-in-Chief of the
Figures

References
-
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19) [Updated 2020 Oct 4]. In: StatPearls [Internet] StatPearls Publishing; 2020. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

