Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events
- PMID: 33852162
- PMCID: PMC8095067
- DOI: 10.1002/14651858.CD007694.pub3
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events
Abstract
Background: Asthma is characterised by chronic inflammation of the airways and recurrent exacerbations with wheezing, chest tightness, and cough. Treatment with inhaled steroids and bronchodilators can result in good control of symptoms, prevention of further morbidity, and improved quality of life. However, an increase in serious adverse events with the use of both regular formoterol and regular salmeterol (long-acting beta₂-agonists) compared with placebo for chronic asthma has been demonstrated in previous Cochrane Reviews. This increase was statistically significant in trials that did not randomise participants to an inhaled corticosteroid, but not when formoterol or salmeterol was combined with an inhaled corticosteroid. The confidence intervals were found to be too wide to ensure that the addition of an inhaled corticosteroid renders regular long-acting beta₂-agonists completely safe; few participants and insufficient serious adverse events in these trials precluded a definitive decision about the safety of combination treatments.
Objectives: To assess risks of mortality and non-fatal serious adverse events in trials that have randomised patients with chronic asthma to regular formoterol and an inhaled corticosteroid versus regular salmeterol and an inhaled corticosteroid.
Search methods: We searched the Cochrane Airways Register of Trials, CENTRAL, MEDLINE, Embase, and two trial registries to identify reports of randomised trials for inclusion. We checked manufacturers' websites and clinical trial registers for unpublished trial data, as well as Food and Drug Administration (FDA) submissions in relation to formoterol and salmeterol. The date of the most recent search was 24 February 2021.
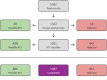
Selection criteria: We included controlled clinical trials with a parallel design, recruiting patients of any age and severity of asthma, if they randomised patients to treatment with regular formoterol versus regular salmeterol (each with a randomised inhaled corticosteroid) and were of at least 12 weeks' duration.
Data collection and analysis: Two review authors independently selected trials for inclusion in the review, extracted outcome data from published papers and trial registries, and applied GRADE rating for the results. We sought unpublished data on mortality and serious adverse events from study sponsors and authors. The primary outcomes were all cause mortality and non-fatal serious adverse events. We chose not to calculate an average result from all the formulations of formoterol and inhaled steroid, as the doses and delivery devices are too diverse to assume a single class effect.
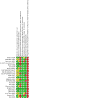
Main results: Twenty-one studies in 11,572 adults and adolescents and two studies in 723 children met the eligibility criteria of the review. No data were available for two studies; therefore these were not included in the analysis. Among adult and adolescent studies, seven compared formoterol and budesonide to salmeterol and fluticasone (N = 7764), six compared formoterol and beclomethasone to salmeterol and fluticasone (N = 1923), two compared formoterol and mometasone to salmeterol and fluticasone (N = 1126), two compared formoterol and fluticasone to salmeterol and fluticasone (N = 790), and one compared formoterol and budesonide to salmeterol and budesonide (N = 229). In total, five deaths were reported among adults, none of which was thought to be related to asthma. The certainty of evidence for all-cause mortality was low, as there were not enough deaths to permit any precise conclusions regarding the risk of mortality on combination formoterol versus combination salmeterol. In all, 201 adults reported non-fatal serious adverse events. In studies comparing formoterol and budesonide to salmeterol and fluticasone, there were 77 in the formoterol arm and 68 in the salmeterol arm (Peto odds ratio (OR) 1.14, 95% confidence interval (CI) 0.82 to 1.59; 5935 participants, 7 studies; moderate-certainty evidence). In the formoterol and beclomethasone studies, there were 12 adults in the formoterol arm and 13 in the salmeterol arm with events (Peto OR 0.94, 95% CI 0.43 to 2.08; 1941 participants, 6 studies; moderate-certainty evidence). In the formoterol and mometasone studies, there were 18 in the formoterol arm and 11 in the salmeterol arm (Peto OR 1.02, 95% CI 0.47 to 2.20; 1126 participants, 2 studies; moderate-certainty evidence). One adult in the formoterol and fluticasone studies in the salmeterol arm experienced an event (Peto OR 0.05, 95% CI 0.00 to 3.10; 293 participants, 2 studies; low-certainty evidence). Another adult in the formoterol and budesonide compared to salmeterol and budesonide study in the formoterol arm had an event (Peto OR 7.45, 95% CI 0.15 to 375.68; 229 participants, 1 study; low-certainty evidence). Only 46 adults were reported to have experienced asthma-related serious adverse events. The certainty of the evidence was low to very low due to the small number of events and the absence of independent assessment of causation. The two studies in children compared formoterol and fluticasone to salmeterol and fluticasone. No deaths and no asthma-related serious adverse events were reported in these studies. Four all-cause serious adverse events were reported: three in the formoterol arm, and one in the salmeterol arm (Peto OR 2.72, 95% CI 0.38 to 19.46; 548 participants, 2 studies; low-certainty evidence).
Authors' conclusions: Overall, for both adults and children, evidence is insufficient to show whether regular formoterol in combination with budesonide, beclomethasone, fluticasone, or mometasone has a different safety profile from salmeterol in combination with fluticasone or budesonide. Five deaths of any cause were reported across all studies and no deaths from asthma; this information is insufficient to permit any firm conclusions about the relative risks of mortality on combination formoterol in comparison to combination salmeterol inhalers. Evidence on all-cause non-fatal serious adverse events indicates that there is probably little to no difference between formoterol/budesonide and salmeterol/fluticasone inhalers. However events for the other formoterol combination inhalers were too few to allow conclusions. Only 46 non-fatal serious adverse events were thought to be asthma related; this small number in addition to the absence of independent outcome assessment means that we have very low confidence for this outcome. We found no evidence of safety issues that would affect the choice between salmeterol and formoterol combination inhalers used for regular maintenance therapy by adults and children with asthma.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
OOS: none known.
ES: none known.
CJC: none known.
Figures



















Update of
-
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events.Cochrane Database Syst Rev. 2010 Jan 20;(1):CD007694. doi: 10.1002/14651858.CD007694.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2021 Apr 14;4:CD007694. doi: 10.1002/14651858.CD007694.pub3. PMID: 20091646 Free PMC article. Updated.
References
References to studies included in this review
Aalbers 2004 {published data only}
-
- Aalbers R, Backer V, Kava TT, Welte T, Omenaas ER, Bergqvist PBF, et al. Adjustable dosing with budesonide/formoterol reduces the rate of asthma exacerbations compared with fixed dosing salmeterol/fluticasone. European Respiratory Journal 2003;41:2-20.
-
- Aalbers R, Backer V, Kava TTK, Omenaas ER, Sandstrom T, Jorup C, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Current Medical Research and Opinion 2004;20(2):225-40. - PubMed
-
- Aalbers R, Harris A, Naya I. Adjustable dosing with budesonide/formoterol achieves sustained guideline ‘well-controlled asthma‘ following step down in treatment. European Respiratory Journal 2005;26(Suppl 49):50s.
-
- Aalbers R, Welte T, Jorup C. Adjustable maintenance dosing (AMD) with budesonide /formoterol (B/F) meets guideline-defined management goals more effectively than fixed dosing (FD) with B/F or salmeterol/fluticasone (S/FL). European Respiratory Journal 2004;24(Suppl 48):311s.
-
- Aalbers R. Fixed or adjustable maintenance-dose budesonide/formoterol compared with fixed maintenance-dose salmeterol fluticasone propionate in asthma patients aged [greater-than or equal to]16 years: post hoc analysis of a randomised, double-blind open-label extension, parallel-group study. Clinical Drug Investigation 2010;30(7):439-51. - PubMed
Akamatsu 2014 {published data only}
-
- Akamatsu T, Shirai T, Kato M, Yasui H, Hashimoto D, Fujisawa T, et al. Switching from salmeterol/fluticasone to formoterol/budesonide combinations improves peripheral airway/alveolar inflammation in asthma. Pulmonary Pharmacology and Therapeutics 2014;27(1):52-6. - PubMed
-
- UMIN000009619. Treatment effect of switching from salmeterol/fluticasone to formoterol/budesonide combinations in patients with asthma. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000011286 (first received 25 December 2012).
Bernstein 2011 {published data only}
-
- Bernstein D, Murphy K, Nolte H. Efficacy comparison of mometasone furoate/formoterol versus fluticasone propionate/salmeterol combination therapies in subjects with persistent asthma: noninferiority and onset-of-action findings. Breast 2019;44:S62.
-
- Bernstein D, Murphy K, Nolte H. Efficacy comparison of mometasone furoate/formoterol versus fluticasone propionate/salmeterol combination therapies in subjects with persistent asthma: noninferiority and onset-of-action findings. World Allergy Organization Journal 2012;5:S79.
-
- Bernstein DI, Hebert J, Cheema A, Murphy KR, Cherrez-Ojeda I, Matiz-Bueno CE, et al. Efficacy and onset of action of mometasone furoate/formoterol and fluticasone propionate/salmeterol combination treatment in subjects with persistent asthma. Allergy, Asthma, and Clinical Immunology 2011;7(1):21. - PMC - PubMed
-
- Bernstein DI, Murphy KR, Nolte H. Non-inferiority efficacy comparison of mometasone furoate/formoterol versus fluticasone propionate/salmeterol combination therapies in subjects with persistent asthma. Allergy, Asthma, and Clinical Immunology 2010;6:33.
-
- EUCTR-004169-33-SK. A 52-week efficacy and safety non-inferiority study of fluticasone propionate/salmeterol 250/50 mcg bid delivered by dry powder inhaler (DISKUS®) versus mometasone furoate/formoterol fumarate 200/10 mcg bid delivered by pressurized metered-dose inhaler in persistent asthmatics previously treated with medium doses of inhaled glucocorticosteroids. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-004169-33-SK (first received 10 April 2008).
Bodzenta‐Lukaszyk 2011 {published data only}
-
- Aalbers R, Brusselle G, McIver T, Grothe B, Bodzenta-Lukaszyk A. Fluticasone/formoterol provides a more rapid bronchodilation than fluticasone/salmeterol over 12 weeks of treatment in patients with asthma. In: 6th IPCRG World Conference; 2012 April 25-28; Edinburgh. 2012:Abstract 269.
-
- Aalbers R, Brusselle G, McIver T, Grothe B, Bodzenta-Lukaszyk A. Onset of bronchodilation with fluticasone/formoterol combination versus fluticasone/salmeterol in an open-label, randomized study. Advances in Therapy 2012;29(11):958-69. - PubMed
-
- Bodzenta-Lukaszyk A, Dymek A, Mansikka H. Fluticasone propionate/formoterol fumarate combination therapy has a more rapid onset of action than fluticasone propionate/salmeterol xinafoate in the treatment of asthma: a randomised controlled trial. Thorax 2010;65(Suppl 4):P24.
-
- Bodzenta-Lukaszyk A, Dymek A, Mansikka H. Fluticasone propionate/formoterol fumarate combination therapy is as effective as fluticasone propionate/salmeterol xinafoate, but has a more rapid onset of action in the treatment of asthma. In: European Respiratory Society Annual Congress; 2010 September 18-22; Barcelona. 2010:[P1205].
-
- Bodzenta-Lukaszyk A, Dymek A, Mansikka H. Fluticasone propionate/formoterol fumarate combination therapy is as effective as fluticasone propionate/salmeterol xinafoate in the treatment of asthma: a randomised controlled trial [Abstract]. Thorax 2010;65(Suppl 4):P177.
Busse 2008 {published data only}
-
- Ambrose H, Lawrance R, Goldman M. Beta-adrenergic receptor gly16arg variation: effect on response to budesonide/formoterol or fluticasone propionate/salmeterol in asthma patients. Chest 2007;132(4):478a.
-
- AstraZeneca D5896C00005. A two-stage randomized, open-label, parallel group, phase III, multicenter, 7 month study to assess the efficacy and safety of Symbicort pMDI administered either as fixed or as an adjustable regimen versus a fixed regimen of Advair in subjects 12 years of age and older with asthma. astrazenecagrouptrials.pharmacm.com/ST/Submission/View?id=1583 (first received 1 November 2003).
-
- Busse WW, Shah SR, Somerville L, Martin P, Goldman M. Comparison of asthma exacerbations and lung function with adjustable-dose budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI), fixed-dose BUD/FM pMDI, and fixed-dose fluticasone/salmeterol dry powder inhaler (FP/SM DPI). www.abstracts2view.com/ats07 (accessed prior to 11 October 2020):A191.
-
- Busse WW, Shah SR, Somerville L, Parasuraman B, Martin P, Goldman M. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. Journal of Allergy and Clinical Immunology 2008;121(6):1407-14. - PubMed
-
- NCT00646594. Atlantis Symbicort [A two stage randomized, open-label, parallel group, phase III, multicenter, 7 month study to assess the efficacy & safety of SYMBICORT pMDI adminstered either as fixed or as an adjustable regimen versus a fixed regimen of Advair in subjects 12 yrs of age and older with asthma]. clinicaltrials.gov/show/NCT00646594 (first received 28 March 2008).
Dahl 2006 {published data only}
-
- Dahl R, Chuchalin A, Gor D, Yoxall S, Sharma R. EXCEL: a randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respiratory Medicine 2006;100(7):1152-62. - PubMed
-
- Dahl R, Chuchalin A, Lindberg A, Jones M, Aggarwal K, Gor D. EXCEL regular maintenance therapy with salmeterol/fluticasone propionate combination (SFC) reduces exacerbations more effectively than the formoterol/budesonide combination (FBC). European Respiratory Journal 2004;24(Suppl 48):309s.
-
- Dahl R, Chuchalin A, Ringdal N, Gor D, Jones M. Salmeterol/fluticasone (SFC) reduces moderate/severe exacerbations more effectively than formoterol/budesonide (FBC) with sustained maintenance therapy EXCEL. In: American Thoracic Society 2005 International Conference; 2005 May 20-25; San Diego. 2005:[C33] [Poster: F68].
-
- GlaxoSmithKline SAM40040. A twenty-four week, randomised, double-dummy, double-blind, parallel group study to compare the rate of asthma exacerbations between SERETIDE DISKUS 50/250ìg 1 inhalation bd and formoterol/budesonide Breath-Actuated Dry Powder Inhaler (BADPI) 4.5/160ìg 2 inhalations bd in subjects with moderate to severe asthma. www.gsk-studyregister.com/en/trial-details/?id=SAM40040 (first received 1 November 2001).
Emeryk 2016 {published data only}
-
- EUCTR-005928-16-FR. An open, randomised, parallel group, multicentre study to compare the efficacy and safety of FlutiForm pMDI vs Seretide pMDI in paediatric subjects with mild to moderate persistent, reversible asthma. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-005928-16-FR (first received 26 March 2007).
-
- McIver T, Emeryk A, Klink R, Schwab B. Fluticasone propionate/formoterol fumarate (FLUT/FORM) combination therapy has comparable efficacy to fluticasone propionate/salmeterol xinafoate ( FLUT/SAL) in paediatric patients with asthma. In: European Respiratory Society Annual Congress; 2011 Sep 24-28; Amsterdam,. 2011:882s [P4822].
EUCTR‐002587‐99‐CZ {published data only}
-
- EUCTR-002587-99-CZ. A 24-week, multicenter, multinational, randomized, double-blind, triple-dummy, 3-arm parallel group study comparing the efficacy and safety of CHF 1535 200/6 (beclomethasone dipropionate 200 µg plus formoterol 6 µg/actuation), 2 puffs b.i.d., versus beclomethasone dipropionate HFA (250 µg/actuation), 4 puffs b.i.d., versus Seretide® 500/50 (fluticasone 500 µg plus salmeterol 50 µg/actuation), 1 inhalation b.i.d., in patients with severe asthma. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-002587-99-CZ (first received 13 September 2007).
EUCTR‐003449‐17‐IT {published data only}
-
- EUCTR-003449-17-IT. A study, 12 weeks, conducted in more centers, treatment with randomly assigned, double-blind, ''double-dummy'', in two parallel groups to compare the efficacy and safety of the combination of Foster NEXThaler, 2 inhalations bid, compared to Seretide Accuhaler, 1 inhalation, the derived parameters of small airways in patients with asthma. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011-003449-17-IT (first received 31 May 2012).
-
- NCT01570478. A study in patients with asthma (NELSON) [A 12-week, multicenter, randomized, double-blind, double-dummy, 2-arm parallel group study comparing the efficacy and safety of Foster® NEXThaler® (beclomethasone dipropionate 100 µg plus formoterol 6 µg/actuation), 2 inhalations b.i.d., versus Seretide® Accuhaler® (fluticasone 250 µg plus salmeterol 50 µg/actuation), 1 inhalation b.i.d., on small airway derived parameters in patients with asthma]. clinicaltrials.gov/show/NCT01570478 (first received 4 April 2012).
EUCTR‐004833‐70‐BG {published data only}
-
- EUCTR-004833-70-BG. A phase III, randomized, parallel group, open study to compare the therapeutic efficacy and safety of SMB budesonide-salmeterol DPI capsule 150/25µg BID delivered by the Axahaler® versus Symbicort® Turbuhaler® 200/12µg BID over 12 weeks in moderate to severe persistent asthmatic patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-004833-70-BG (first received 19 January 2009).
Hsieh 2013 {published data only}
-
- Hsieh M-J, Lai R-S, Wu C-L, Lai C-L, Wang C-C, Peng D-W, et al. A double-blind, double-dummy, randomized study of beclomethasone/formoterol versus fluticasone/salmeterol in treatment of moderate to severe asthma in Taiwan. European Respiratory Journal 2013;42:873s [P4120].
-
- Hsieh MJ, Lin YC, Lai RS, Wu CL, Lai CL, Wang CC, et al. Comparative efficacy and tolerability of beclomethasone/formoterol and fluticasone/salmeterol fixed combination in Taiwanese asthmatic patients. Journal of the Formosan Medical Association 2017;12:1078-85. - PubMed
Kuna 2007 {published data only}
-
- Ambrose H, Lawrance R, Goldman M. Beta-adrenergic receptor gly16arg variation: effect on response to budesonide/formoterol or fluticasone propionate/salmeterol in asthma patients. Chest 2007;132(4):478a.
-
- AstraZeneca (SD-0390735). Comparison of the efficacy and safety of one inhalation of Symbicort®Turbuhaler® 160/4.5 μg bid plus as-needed with two inhalations of SeretideTM EvohalerTM 25/125 μg bid plus Terbutaline Turbuhaler® 0.4 mg as-needed, and one inhalation of Symbicort® Turbuhaler® 320/9 μg bid plus Terbutaline Turbuhaler® 0.4 mg as-needed. A 6-month, randomised, double-blind, double-dummy, parallel-group, active-controlled,multicentre, phase IIIB study in adult and adolescent asthmatic patients. www.astrazenecaclinicaltrials.com/_mshost2715844/content/content/resourc... (first recevied 30 April 2009).
-
- Bleecker ER, Postma DS, Lawrance R, Meyers DA, Ambrose H, Goldman M. Effect of polymorphisms in the beta2-adrenergic receptor gene (ADRB2) on response to long-acting beta2-agonist (LABA) therapy. Journal Allergy and Clinical Immunology 2007;119(2):523.
-
- Boonsawat W. Cost-effectiveness of budesonide/formoterol maintenance and rescue therapy in Thailand. Asian Biomedicine 2010;4(4):571-8.
Maspero 2010 {published data only}
-
- FDA. Clinical review of Dulera (NDA 22-518). www.fda.gov/media/100092/download (accessed before 18 November 2020).
-
- Maspero J, Cherrez I, Nolte H. Long-term safety and tolerability of two doses of mometasone furoate/formoterol (MF/F) combination, administered via a metered-dose inhaler, for the treatment of moderate-to-severe persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S159.
-
- Maspero J, Cherrez I, Nolte H. Mometasone furoate/formoterol (mf/f) combination treatment in patients with moderate-to-severe asthma: findings from a 1-yr safety trial [Abstract]. In: European Respiratory Society Annual Congress; 2009 Sep 12-16; Vienna. 2009:[E274].
-
- Maspero J, Nolte H, Cherrez I. Long-term safety of medium- and high-dose mometasone furoate/formoterol combination in persistent asthmatics: analysis of adverse event incidence, plasma cortisol, and ocular changes [Abstract]. In: European Respiratory Society Annual Congress; 2010 Sep 18-22; Barcelona. 2010:[P1211].
-
- Maspero J, Nolte H, Ojeda IC. Long-term safety of medium- and high-doses mometasone furoate/formoterol combination in persistent asthmatics: analysis of adverse events incidence, plasma cortisol, and ocular changes [Abstract]. Journal of Allergy and Clinical Immunology 2010;125(2 Suppl 1):AB197.
NCT00901368 {published data only}
-
- Barnes N, Van Noord JA, Brindicci C, Lindemann L, Varoli G, Perpina M, et al. Maintenance of lung function and asthma control with extrafine beclomethasone/formoterol. European Respiratory Journal 2011;38(55):140s [P833].
-
- Barnes N, Noord JA, Brindicci C, Lindemann L, Varoli G, Perpina M, et al. Stepping-across controlled asthmatic patients to extrafine beclometasone/formoterol combination. Pulmonary Pharmacology and Therapeutics 2013;26(5):555-61. - PubMed
-
- EUCTR-003740-11-ES. A phase 4, multinational, multicentre, double blind, double dummy, randomized, parallel group, controlled clinical study of fixed combination beclomethasone dipropionate 100 µg plus formoterol fumarate 6 µg pmdi with hfa-134a propellant (chf1535, Foster®) versus fluticasone 250 µg plus salmeterol 50 µg dpi (Seretide® Diskus®) as maintenance treatment in controlled asthmatic patients. - facto study [Estudio fase iv, multinacional, multicentrico, doble ciego, doble simulacion, randomizado, de grupos paralelos, controlado, sobre la combinacion de dosis fijas de dipropionato de beclometasona 100 µg mas fumarato de formoterol 6 µg pmdi con propelente hfa-134a (chf1535, foster®) versus fluticasona 250 µg mas salmeterol 50 µg ips (seretide® diskus®) como tratamiento de mantenimiento de pacientes asmaticos controlados]. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-003740-11-ES (first received 23 January 2009).
-
- EUCTR-003740-11-NL. A phase 4, multinational, multicentre, double blind, double dummy, randomized, parallel group, controlled clinical study of fixed combination beclomethasone dipropionate 100 µg plus formoterol fumarate 6 µg pmdi with hfa-134a propellant (chf1535, Foster®) versus fluticasone 250 µg plus salmeterol 50 µg dpi (Seretide® Diskus®) as maintenance treatment in controlled asthmatic patients. - facto study. www.clinicaltrialsregister.eu/ctr-search/trial/2008-003740-11/results (first received 6 January 2017).
-
- NCT00901368. FACTO study (Foster® As Complete Treatment Option) [A phase 4, multinational, multicentre, double blind, double dummy, randomized, parallel group, controlled clinical study of fixed combination beclomethasone dipropionate 100 µg plus formoterol fumarate 6 µg pmdi with hfa-134a propellant (chf1535, foster®) versus fluticasone 250 µg plus salmeterol 50 µg dpi (seretide® diskus®) as maintenance treatment in controlled asthmatic patients]. clinicaltrials.gov/show/NCT00901368 (first received 13 May 2009).
Papi 2007 {published data only}
-
- NCT00394368. Efficacy and tolerability of beclomethasone dipropionate 100 µg + formoterol 6 µg pMDI VIA HFA-134a vs. fluticasone 125 µg + salmeterol 25 µg pMDI [DOUBLE BLIND, MULTINATIONAL, MULTICENTRE, PARALLEL-GROUP DESIGN CLINICAL TRIAL OF THE EFFICACY AND TOLERABILITY OF CHF 1535 (BECLOMETHASONE DIPROPIONATE 100 µg + FORMOTEROL 6 µg) pMDI VIA HFA-134a vs. FLUTICASONE 125 µg + SALMETEROL 25 µg pMDI (SERETIDE®) IN THE 12-WEEK TREATMENT OF ADULT PATIENTS WITH MODERATE TO SEVERE PERSISTENT ASTHMA]. clinicaltrials.gov/show/NCT00394368 (first received 1 November 2006).
-
- Paggiaro P, Papi A, Feschenko YI, Nicolini G, Fabbri LM. Efficacy and safety of the new beclomethasone dipropionate/formoterol combination vs fluticasone propionate/salmeterol pMDIs in moderate to severe persistent asthma. European Respiratory Journal 2006;28(Suppl 50):205s [P1227].
-
- Papi A, Paggiaro P, Nicolini G, Vignola AM, Fabbri LM. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. European Journal of Allergy and Clinical Immunology 2007;62(10):1182-8. - PubMed
Papi 2012 {published data only}
-
- NCT00497237. Clinical trial of the efficacy and safety of beclomethasone dipropionate plus formoterol vs fluticasone propionate plus salmeterol in the 6 months step down treatment of asthma [Prospective, randomised, open-label, multicentre, active drug controlled, parallel group design clinical trial of the efficacy and safety of beclomethasone dipropionate 400 mcg + formoterol 24 mcg pMDI Via HFA-134a (Foster™) vs. fluticasone propionate 500 mcg + salmeterol xinafoate 100 mcg DPI (Seretide Diskus®) in the 6 months stepdown treatment of adult patients with controlled asthma]. clinicaltrials.gov/show/NCT00497237 (first received 6 July 2007).
-
- Paggiaro P, Nicolini G, Crimi N, Fabbri LM, Olivieri D, Rossi A, et al. Asthma control and lung function after step down from high dose ICS/LABA combination therapy. European Respiratory Journal 2011;38(55):722s [P3967].
Ploszczuk 2014 {published data only}
-
- CTRI//05/002704. Comparison of Flutiform, fluticasone and Seretide in treatment of moderate to severe asthma in paediatric patients aged 5 to less than 12 years. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/05/002704 (first received 28 May 2012).
Ringdal 2002 {published data only}
-
- Jenkins C, Wilson J, Rutherford C, Perry AS, Whitehead PJ. Asthma management costs are lower with combination fluticasone/salmeterol (25/50 mcg BD) in a single inhaler than with budesonide (800 mcg BD) plus eformoterol (12 mcg BD) via separate inhalers. Respirology 2002;7(Suppl):A20 Abstract number (P23).
-
- Martin AA, Whitehead PJ, McCarthy TP. Asthma costs with salmeterol/fluticasone combination 50/250mcg bd compared to budesonide 800mcg bd plus formoterol 12mcg bd [abstract]. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. 2003:D034 Poster C43.
-
- Ringdal N, Chovan L, Chuchalin AG, Whitehead PJ. Advair™/Seretide™ (250µg/50µg bid) shows exacerbation benefit over budesonide 800µg + formoterol 12µg in moderate-severe asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A866.
-
- Ringdal N, Chuchalin A, Chovan L, Tudoric N, Maggi E, Whitehead PJ, et al. Evaluation of different inhaled combination therapies (EDICT): a randomised, double-blind comparison of Seretide (50/250 mug bd Diskus vs. formoterol (12 mug bd) and budesonide (800 mug bd) given concurrently (both via Turbuhaler) in patients with moderate-to-severe asthma. Respiratory Medicine 2002;96(11):851-61. - PubMed
-
- Ringdal N, Chuchalin AG, Chovan L, Whitehead PJ. A comparison of Advair™/Seretide™ (salmeterol 50 µg/fluticasone propionate 250 µg bid) with formoterol 12 µg + budesonide 800 µg bid in moderate-severe asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A196.
SAM 40010 {published data only}
-
- SAM40010. A randomised, double blind, double-dummy, parallel-group, twelve week comparison of salmeterol/fluticasone propionate (FP) DISKUS/ACCUHALER 50/100mcg bd. with budesonide 200mcg bd. plus formoterol 4.5mcg bd. (both by breath-activated dry powder inhaler [BADPI]) in adult and adolescent asthmatics. www.gsk-studyregister.com/en/trial-details/?id=SAM40010 (first received 26 January 2000).
SAM 40048 {published data only}
-
- SAM40048. Randomised, double-blind, parallel group study on the efficacy and tolerability of the salmeterol 50 mcg/fluticasone 250 mcg combination Diskus compared to the formoterol 6mcg / budesonide 200mcg combination Turbohaler administered twice daily in patients with moderate bronchial asthma. GlaxoSmithKline Clinical Trial Register (first received 16 August 2001).
Scichilone 2010 {published data only}
-
- EUCTR-000512-25-IT. Double blind, double dummy, randomised, parallel group, monocentric clinical trial on the effects of chf 1535 mdi or seretide dpi on biological and functional markers of small airways in patients with asthma: a pilot study - nd. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-000512-25-IT (first received 20 April 2006).
-
- Scichilone N, Battaglia S, Sorino C, Paglino G, Martino L, Paterno A, et al. Effects of extra-fine inhaled beclomethasone/formoterol on both large and small airways in asthma. Allergy 2010;65(7):897-902. - PubMed
Usmani 2017 {published data only}
-
- Kemppinen A, Gardener E, Thomas V, Raju P, Callan C, McLoughlin A, et al. Pragmatic trial stepping down Flutiform in patients maintained on high dose ICS. Journal of Thoracic Disease 2016;8(Suppl 5):AB039.
-
- Usmani OS, Kemppinen A, Gardener E, Thomas V, Konduru PR, Callan C, et al. A randomized pragmatic trial of changing to and stepping down fluticasone/formoterol in asthma. Journal of Allergy and Clinical Immunology 2017;5(5):1378-87.e5. - PubMed
Woo 2020 {published data only}
References to studies excluded from this review
Bleecker 2008 {published data only}
-
- Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to long acting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet 2008;370(9605):2118-25. - PubMed
Dhillon 2006 {published data only}
-
- Dhillon S, Keating GM. Beclometasone dipropionate/formoterol in an HFA-propelled pressurised metered-dose inhaler. Drugs 2006;66(11):1475-83. - PubMed
Hampel 2008 {published data only}
-
- Hampel FC, Martin P, Mezzanotte WS. Early bronchodilatory effects of budesonide/formoterol pMDI compared with fluticasone/salmeterol DPI and albuterol pMDI: 2 randomized controlled trials in adults with persistent asthma previously treated with inhaled corticosteroids. Journal of Asthma 2008;45(4):265-72. - PubMed
Jung 2008 {published data only}
-
- Jung KS, Uh ST, Lee YC, Shim JJ, Park SK, Williams AE, et al. Comparison of the clinical efficacy and safety of salmeterol/fluticasone propionate versus current care in the management of persistent asthma in Korea. Current Medical Research and Opinion 2008;24(12):3571-82. - PubMed
Lee 2003 {published data only}
-
- Lee DKC, Currie GP, Cockburtn WJ, Lipworth BJ. Comparison of budesonide/formoterol versus fluticasone/salmeterol combination inhalers in moderate persistent asthma. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. 2003:D094 Poster 613.
Lyseng‐Williamson 2003 {published data only}
-
- Lyseng-Williamson KA, Plosker GL. Inhaled salmeterol/fluticasone propionate combination: a pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics 2003;21(13):951-89. - PubMed
NCT02491970 {published data only}
-
- NCT02491970. Small airway function of fluticasone/formoterol (Flutiform®) and fluticasone/salmeterol (RECONFFIRM) [A single-blind, randomized, active-controlled, multi-center and phase IV study to evaluate the small airway parameters of fluticasone/formoterol (Flutiform®) compared to fluticasone/salmeterol in asthma patients]. clinicaltrials.gov/show/NCT02491970 (first received 8 July 2015).
Rani 2016 {published data only}
-
- Rani S, Rawal M, Kumar S, Lamba S. To compare efficacy and safety of fixed drug combination of salmeterol / fluticasone and budesonide / formoterol on the lung functions in childhood patients with moderate persistent asthma. Indian Journal of Public Health Research and Development 2016;7(4):203-7.
UMIN000006572 {published data only}
-
- JPRN-UMIN000006572. Study on the effects of the budesonide/formoterol combination formulation in improving airway inflammation and airway resistance in patients with bronchial asthma. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000006572 (first received 18 October 2011).
References to ongoing studies
NCT03387241 {published data only}
-
- NCT03387241. Efficacy of FLUTIFORM ® vs Seretide® in moderate to severe persistent asthma in subjects aged ≥12 years [A double blind, double dummy, randomised, multicentre, two arm parallel group study to assess the efficacy and safety of FLUTIFORM® pMDI (2 puffs bid) vs Seretide® pMDI (2 puffs bid) in subjects aged ≥12 years with moderate to severe persistent, reversible asthma]. ClinicalTrials.gov/show/NCT03387241 (first received 2 January 2018).
Additional references
Altman 2003
Anderson 2006
-
- Anderson GP. Current issues with beta(2)-adrenoceptor agonists - pharmacology and molecular and cellular mechanisms. Clinical Reviews in Allergy and Immunology 2006;31(2-3):119-30. - PubMed
Arnold 1985
Barnes 1993
-
- Barnes PJ. Beta-adrenoceptors on smooth-muscle, nerves and inflammatory cells. Life Sciences 1993;52(26):2101-9. - PubMed
Barnes 1995
-
- Barnes PJ. Beta-adrenergic receptors and their regulation. American Journal of Respiratory and Critical Care Medicine 1995;152(3):838-60. - PubMed
Barnes 2002
-
- Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. European Respiratory Journal 2002;19:182-91. - PubMed
Bennett 1994
Bijl‐Hofland 2001
-
- Bijl-Hofland ID, Cloosterman SG, Folgering HT, Van den Elshout FJ, Van Weel C, Van Schayck CP. Inhaled corticosteroids, combined with long-acting beta(2)-agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory and Critical Care Medicine 2001;164(5):764-9. - PubMed
Brown 1983
-
- Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta-2-receptor stimulation by circulating epinephrine. New England Journal of Medicine 1983;309(23):1414-9. - PubMed
Burgess 1991
-
- Burgess CD, Windom HH, Pearce N, Marshall S, Beasley R, Siebers RWL, et al. Lack of evidence for beta-2 receptor selectivity - a study of metaproterenol, fenoterol, isoproterenol, and epinephrine in patients with asthma. American Review of Respiratory Disease 1991;143(2):444-6. - PubMed
Burggraaf 2001
Cates 2008
Cates 2008a
Cates 2012
Cates 2018
Cochrane Airways 2019
-
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 7 May 2019).
Cockcroft 1993
-
- Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993;342(8875):833-7. - PubMed
Collins 1969
Crane 1989
-
- Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981-83 - case-control study. Lancet 1989;1(8644):917-22. - PubMed
Ducharme 2010
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD005533. [DOI: 10.1002/14651858.CD005533.pub2] - DOI - PMC - PubMed
Ducharme 2011
Giembycz 2006
-
- Giembycz MA, Newton R. Beyond the dogma: novel ß2-adrenoceptor signalling in the airways. European Respiratory Journal 2006;27(6):1286-306. - PubMed
Grainger 1991
Guhan 2000
Hall 1989
-
- Hall JA, Petch MC, Brown MJ. Intracoronary injections of salbutamol demonstrate the presence of functional beta-2-adrenoceptors in the human-heart. Circulation Research 1989;65(3):546-53. - PubMed
Hanania 2002
-
- Hanania NA, Sharfkhaneh A, Barber R, Dickey BF. Beta-agonist intrinsic efficacy - measurement and clinical significance. American Journal of Respiratory and Critical Care Medicine 2002;165(10):1353-8. - PubMed
Hanania 2007
-
- Hanania NA, Moore RH, Zimmerman JL, Miller CT, Bag R, Sharafkhaneh A, et al. The role of intrinsic efficacy in determining response to beta(2)-agonist in acute severe asthma. Respiratory Medicine 2007;101(5):1007-14. - PubMed
Hancox 1999
-
- Hancox RJ, Aldridge RE, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, et al. Tolerance to beta-agonists during acute bronchoconstriction. European Respiratory Journal 1999;14(2):283-7. - PubMed
Hancox 2006
-
- Hancox RJ. Interactions between corticosteroids and beta2-agonists. Clinical Reviews in Allergy and Immunology 2006;31(2-3):231-46. - PubMed
Haney 2006
-
- Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clinical Reviews in Allergy and Immunology 2006;31(2-3):181-96. - PubMed
Harvey 1982
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org 2011.
Higgins 2019
-
- Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. 2019:143-76.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 [updated September 2020]. Cochrane, 2020. Available from training.cochrane.org/handbook.
ICHE2A
-
- Clinical safety data management: definitions and standards for expedited reporting. www.ema.europa.eu/en/ich-e2a-clinical-safety-data-management-definitions... (accessed before 19 November 2020).
Janjua 2019
Jones 2001
-
- Jones SL, Cowan JO, Flannery EM, Hancox RJ, Herbison GP, Taylor DR. Reversing acute bronchoconstriction in asthma: the effect of bronchodilator tolerance after treatment with formoterol. European Respiratory Journal 2001;17(3):368-73. - PubMed
Lasserson 2008
-
- Lasserson TJ, Cates CJ, Ferrara G, Casali L. Combination fluticasone and salmeterol versus fixed dose combination budesonide and formoterol for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004106. [DOI: 10.1002/14651858.CD004106.pub3] - DOI - PubMed
Lee 2003a
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4. Searching for studies. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 [updated September 2020]. Cochrane, 2020.
Lipworth 1989
-
- Lipworth BJ, Struthers AD, McDevitt DG. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high-dose inhaled salbutamol in asthmatics. American Review of Respiratory Disease 1989;140(3):586-92. - PubMed
Lipworth 1992
Lipworth 1997
-
- Lipworth BJ. Airway sub-sensitivity with long-acting beta 2-agonists: is there cause for concern? Drug Safety 1997;16(5):295-308. - PubMed
Lipworth 2000
-
- Lipworth BJ, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest 2000;117(1):156-62. - PubMed
Lotvall 2001
Marshall 2018
McDevitt 1974
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town, South Africa. 2017.
McIvor 1998
-
- McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 1998;158(3):924-30. - PubMed
Morrison 1993
-
- Morrison KJ, Gao Y, Vanhoutte PM. Beta-adrenoceptors and the epithelial layer in airways. Life Sciences 1993;52(26):2123-30. - PubMed
Nelson 1977
-
- Nelson HS, Raine D, Doner HC, Posey WC. Subsensitivity to bronchodilator action of albuterol produced by chronic administration. American Review of Respiratory Disease 1977;116(5):871-8. - PubMed
Ni Chroinin 2009
-
- Ni Chroinin M, Greenstone I, Lasserson TJ, Ducharme FM. Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD005307. [DOI: 10.1002/14651858.CD005307] - DOI - PMC - PubMed
Ni Chroinin 2010
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD005535. [DOI: 10.1002/14651858.CD005535.pub2] - DOI - PMC - PubMed
Noel‐Storr 2018
-
- Noel-Storr AH, Project Transform Team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK.
Ouzzani 2016
Palmqvist 1999
-
- Palmqvist M, Ibsen T, Mellen A, Lotvall J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. American Journal of Respiratory and Critical Care Medicine 1999;160(1):244-9. - PubMed
Pearce 1990
Pearce 2007
-
- Pearce N. Adverse Reactions: The Fenoterol Story. 1st edition. Auckland: Auckland University Press, 2007. [9781869403744]
Peters 2016
-
- Peters SP, Bleecker ER, Canonica GW, Park YB, Ramirez R, Hollis S, et al. Serious asthma events with budesonide plus formoterol vs. budesonide alone. New England Journal of Medicine 2016;375(9):850-60. - PubMed
RevMan 5 [Computer program]
-
- The Nordic Cochrane Centre: The Cochrane Collaboration Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Salpeter 2006
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Annals of Internal Medicine 2006;144(12):904-12. - PubMed
Sears 1990
-
- Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, et al. Regular inhaled beta-agonist treatment in bronchial-asthma. Lancet 1990;336(8728):1391-6. - PubMed
SMART 2006
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15-26. - PubMed
Speizer 1968
Stempel 2016
Stempel 2016a
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
van der Woude 2001
vanNoord 1996
-
- vanNoord JA, Smeets JJ, Raaijmakers JAM, Bommer AM, Maesen FPV. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684-8. - PubMed
Walters 2002
-
- Walters EH, Walters JAE, Gibson PW. Regular treatment with long acting beta agonists versus daily regular treatment with short acting beta agonists in adults and children with stable asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003901. [DOI: 10.1002/14651858.CD003901] - DOI - PMC - PubMed
Walters 2007
Weber 1982
-
- Weber RW, Smith JA, Nelson HS. Aerosolized terbutaline in asthmatics - development of subsensitivity with long-term administration. Journal of Allergy and Clinical Immunology 1982;70(6):417-22. - PubMed
Weinstein 2019
Wilson 1981
-
- Wilson JD, Sutherland DC, Thomas AC. Has the change to beta-agonists combined with oral theophylline increased cases of fatal asthma. Lancet 1981;1(8232):1235-7. - PubMed
Wong 1990
-
- Wong CS, Pavord ID, Williams J, Britton JR, Tattersfield AE. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet 1990;336(8728):1396-9. - PubMed
Yates 1996
-
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long-acting inhaled beta(2)-agonist. American Journal of Respiratory and Critical Care Medicine 1996;154(6):1603-7. - PubMed
References to other published versions of this review
Cates 2009
-
- Cates CJ, Lasserson T. Regular fixed-dose treatment with formoterol and inhaled corticosteroids versus regular treatment with salmeterol and inhaled corticosteroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007694. [DOI: 10.1002/14651858.CD007694] - DOI - PMC - PubMed
Cates 2010
-
- Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD007694. [DOI: 10.1002/14651858.CD007694.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

