PARP Inhibitor in Platinum-Resistant Ovarian Cancer: Single-Center Real-World Experience
- PMID: 33852339
- PMCID: PMC8162970
- DOI: 10.1200/GO.20.00269
PARP Inhibitor in Platinum-Resistant Ovarian Cancer: Single-Center Real-World Experience
Abstract
Purpose: Poly (ADP-ribose) polymerase inhibitors (PARPi) have proven efficacy in treatment of BReast CAncer (BRCA) gene mutation-positive platinum-sensitive ovarian cancers. There is paucity of data for their role in platinum-resistant ovarian cancer (PROC). We report here retrospective analysis of outcome of PARPi treatment in a group of patients including those of PROC.
Patients and methods: We analyzed all consecutive patients who received PARPi. The efficacy of PARPi monotherapy was assessed in patients with relapsed high-grade serous ovarian carcinoma with gBRCAm. The drug was procured through compassionate program. Drugs (olaparib and talazoparib) were provided in capsule form.
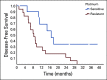
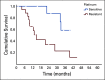
Results: Between July 1, 2015, and June 30, 2019, 28 patients with ovarian cancer received PARPi. At the time of data censoring (September 30, 2019), four (14.3%) patients are still on treatment. Median age was 54.5 years (range, 39-75 years). Median number of previous lines of chemotherapy received was three (range, 1-6). Eleven platinum-sensitive patients received the drug as maintenance (five in complete response and six in partial response after chemotherapy), whereas 17 (60.7%) had platinum-resistant progressive disease while starting the drug. In PROC, objective response rate (complete response plus partial response) was 47%, median progression-free survival was 8.2 months (5.3-11.3), and overall survival was 14.9 months (11.2-18.5). No new side effects were observed.
Conclusion: This is the first study from India evaluating PARPi in platinum-resistant ovarian cancer. This study suggests that PARPi is a viable treatment option in patients with PROC with gBRCAm. This should be further evaluated in randomized clinical trial.
Conflict of interest statement
Figures
References
-
- du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer J Natl Cancer Inst 951320–13292003 - PubMed
-
- Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer J Clin Oncol 183084–30922000 - PubMed
-
- Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group Study J Clin Oncol 213194–32002003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical