Polyester nasal swabs collected in a dry tube are a robust and inexpensive, minimal self-collection kit for SARS-CoV-2 testing
- PMID: 33852576
- PMCID: PMC8046217
- DOI: 10.1371/journal.pone.0245423
Polyester nasal swabs collected in a dry tube are a robust and inexpensive, minimal self-collection kit for SARS-CoV-2 testing
Abstract
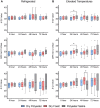
Background: In order to identify an inexpensive yet highly stable SARS-CoV-2 collection device as an alternative to foam swabs stored in transport media, both contrived ("surrogate") CoV-positive and patient-collected spun polyester swabs stored in dry tubes were evaluated for time- and temperature-stability using qPCR.
Methods: Surrogate specimens were prepared by combining multiple, residual SARS-CoV-2-positive clinical specimens and diluting to near-LOD levels in either porcine or human mucus ("matrix"), inoculating foam or polyester nasal swabs, and sealing in dry tubes. Swabs were then subjected to one of three temperature excursions: (1) 4°C for up to 72 hours; (2) 40°C for 12 hours, followed by 32°C for up to 60 hours; or (3) multiple freeze-thaw cycles (-20°C). The stability of extracted SARS-CoV-2 RNA for each condition was evaluated by qPCR. Separate usability studies for the dry polyester swab-based HealthPulse@home COVID-19 Specimen Collection Kit were later conducted in both adult and pediatric populations.
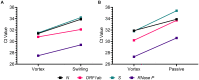
Results: Polyester swabs stored dry demonstrated equivalent performance to foam swabs for detection of low and moderate SARS-CoV-2 viral loads. Mimicking warm- and cold- climate shipment, surrogate specimens were stable following either 72 hours of a high-temperature excursion or two freeze-thaw cycles. In addition, usability studies comprised of self-collected patient specimens yielded sufficient material for molecular testing, as demonstrated by RNase P detection.
Conclusions: Polyester nasal swabs stored in dry collection tubes offer a robust and inexpensive self-collection method for SARS-CoV-2 viral load testing, as viral RNA remains stable under conditions required for home collection and shipment to the laboratory.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interest: LRP, LAK, CLA, DKS, JSE, and DR are employed by Quantigen Biosciences. YT is employed by The Everett Clinic-Part of Optum and MLW is employed by Sciest LLC. These affiliations do not alter our adherence to PLOS ONE policies of sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures



References
-
- Altamirano J, Govindarajan P, Blomkalns AL, Kushner LE, Stevens BA, Pinsky BA, et al. Assessment of sensitivity and specificity of patient-collected lower nasal specimens for sudden acute respiratory syndrome coronavirus 2 testing. JAMA Netw Open. 2020; 3(6):e2012005. 10.1001/jamanetworkopen.2020.12005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

