COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers
- PMID: 33852719
- PMCID: PMC8054201
- DOI: 10.1083/jcb.201907224
COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers
Abstract
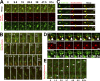
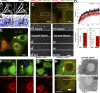
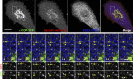
COPII and COPI mediate the formation of membrane vesicles translocating in opposite directions within the secretory pathway. Live-cell and electron microscopy revealed a novel mode of function for COPII during cargo export from the ER. COPII is recruited to membranes defining the boundary between the ER and ER exit sites, facilitating selective cargo concentration. Using direct observation of living cells, we monitored cargo selection processes, accumulation, and fission of COPII-free ERES membranes. CRISPR/Cas12a tagging, the RUSH system, and pharmaceutical and genetic perturbations of ER-Golgi transport demonstrated that the COPII coat remains bound to the ER-ERES boundary during protein export. Manipulation of the cargo-binding domain in COPII Sec24B prohibits cargo accumulation in ERES. These findings suggest a role for COPII in selecting and concentrating exported cargo rather than coating Golgi-bound carriers. These findings transform our understanding of coat proteins' role in ER-to-Golgi transport.
© 2021 Shomron et al.
Figures








Comment in
-
Want to leave the ER? We offer vesicles, tubules, and tunnels.J Cell Biol. 2021 Jun 7;220(6):e202104062. doi: 10.1083/jcb.202104062. Epub 2021 May 17. J Cell Biol. 2021. PMID: 33999114 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

