Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases
- PMID: 33852854
- PMCID: PMC8106763
- DOI: 10.1016/j.celrep.2021.108998
Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases
Abstract
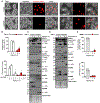
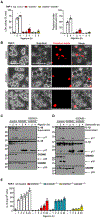
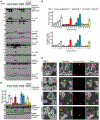
Cellular inflammasome activation causes caspase-1 cleavage of the pore-forming protein gasdermin D (GSDMD) with subsequent pyroptotic cell death and cytokine release. Here, we clarify the ambiguous role of the related family member gasdermin E (GSDME) in this process. Inflammasome stimulation in GSDMD-deficient cells led to apoptotic caspase cleavage of GSDME. Endogenous GSDME activation permitted sublytic, continuous interleukin-1β (IL-1β) release and membrane leakage, even in GSDMD-sufficient cells, whereas ectopic expression led to pyroptosis with GSDME oligomerization and complete liberation of IL-1β akin to GSDMD pyroptosis. We find that NLRP3 and NLRP1 inflammasomes ultimately rely concurrently on both gasdermins for IL-1β processing and release separately from their ability to induce cell lysis. Our study thus identifies GSDME as a conduit for IL-1β release independent of its ability to cause cell death.
Keywords: GSDMD; GSDME; IL-1; cell death; gasdermin D; gasdermin E; gasdermins; inflammasome; interleukin-1; pore-forming proteins.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Broz P, Pelegrín P, and Shao F (2020). The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol 20, 143–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

