A methionine-Mettl3-N6-methyladenosine axis promotes polycystic kidney disease
- PMID: 33852874
- PMCID: PMC8172529
- DOI: 10.1016/j.cmet.2021.03.024
A methionine-Mettl3-N6-methyladenosine axis promotes polycystic kidney disease
Abstract
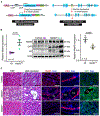
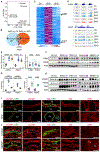
Autosomal dominant polycystic kidney disease (ADPKD) is a common monogenic disorder marked by numerous progressively enlarging kidney cysts. Mettl3, a methyltransferase that catalyzes the abundant N6-methyladenosine (m6A) RNA modification, is implicated in development, but its role in most diseases is unknown. Here, we show that Mettl3 and m6A levels are increased in mouse and human ADPKD samples and that kidney-specific transgenic Mettl3 expression produces tubular cysts. Conversely, Mettl3 deletion in three orthologous ADPKD mouse models slows cyst growth. Interestingly, methionine and S-adenosylmethionine (SAM) levels are also elevated in ADPKD models. Moreover, methionine and SAM induce Mettl3 expression and aggravate ex vivo cyst growth, whereas dietary methionine restriction attenuates mouse ADPKD. Finally, Mettl3 activates the cyst-promoting c-Myc and cAMP pathways through enhanced c-Myc and Avpr2 mRNA m6A modification and translation. Thus, Mettl3 promotes ADPKD and links methionine utilization to epitranscriptomic activation of proliferation and cyst growth.
Keywords: AVPR2; METTL3; N(6)-methyladenosine; S-adenosylmethionine; c-Myc; m6A mRNA methylation; mRNA translation; methionine; polycystic kidney disease.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.N.C has a patent on the use of PAPP-A inhibitors in ADPKD. E.N.C. is a consultant for TeneoBio, Calico, Mitobridge, and Cytokinetics. E.N.C. is on the advisory board of Eolo Pharma. E.N.C. owns stocks in Teneobio. Unrelated to this work, V.P. has patents involving the use of anti-miR-17 for the treatment of ADPKD (16/466,752 and 15/753,865). V.P. has previously held investment interests in Regulus Therapeutics and serves as a consultant for Otsuka Pharmaceuticals. V.P. lab has a sponsored research agreement with Regulus Therapeutics (unrelated to this work).
Figures




Comment in
-
Methionine metabolism: a driver of ADPKD.Nat Rev Nephrol. 2021 Jun;17(6):368. doi: 10.1038/s41581-021-00437-z. Nat Rev Nephrol. 2021. PMID: 33948026 No abstract available.
References
-
- Bedi RK, Huang D, Eberle SA, Wiedmer L, Sledz P, and Caflisch A (2020). Small-Molecule Inhibitors of METTL3, the Major Human Epitranscriptomic Writer. ChemMedChem 15, 744–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

