Antibody evasion by the P.1 strain of SARS-CoV-2
- PMID: 33852911
- PMCID: PMC8008340
- DOI: 10.1016/j.cell.2021.03.055
Antibody evasion by the P.1 strain of SARS-CoV-2
Abstract
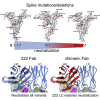
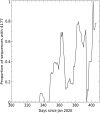
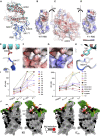
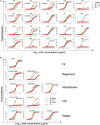
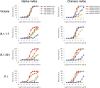
Terminating the SARS-CoV-2 pandemic relies upon pan-global vaccination. Current vaccines elicit neutralizing antibody responses to the virus spike derived from early isolates. However, new strains have emerged with multiple mutations, including P.1 from Brazil, B.1.351 from South Africa, and B.1.1.7 from the UK (12, 10, and 9 changes in the spike, respectively). All have mutations in the ACE2 binding site, with P.1 and B.1.351 having a virtually identical triplet (E484K, K417N/T, and N501Y), which we show confer similar increased affinity for ACE2. We show that, surprisingly, P.1 is significantly less resistant to naturally acquired or vaccine-induced antibody responses than B.1.351, suggesting that changes outside the receptor-binding domain (RBD) impact neutralization. Monoclonal antibody (mAb) 222 neutralizes all three variants despite interacting with two of the ACE2-binding site mutations. We explain this through structural analysis and use the 222 light chain to largely restore neutralization potency to a major class of public antibodies.
Keywords: P.1; RBD; SARS-CoV-2; VH3-53; antibody; escape; neutralization; spike; structure; variant.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.R.S. sits on the GSK Vaccines Scientific Advisory Board. Oxford University holds intellectual property related to the Oxford-AstraZeneca vaccine. A.J.P. is chair of the UK Department Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in the JCVI COVID-19 committee and is a member of the World Health Organization’s (WHO’s) SAGE. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, or WHO. S.C.G. is co-founder of Vaccitech (collaborators in the early development of this vaccine candidate) and is named as an inventor on a patent covering use of ChAdOx1-vectored vaccines and a patent application covering this SARS-CoV-2 vaccine (PCT/GB2012/000467). T.L. is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and was a consultant to Vaccitech for an unrelated project during the conduct of the study. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development.The University of Oxford has protected intellectual property disclosed in this publication.
Figures





References
-
- Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F. Structures of Human Antibodies Bound to SARS-CoV-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies. Cell. 2020;182:828–842.e16. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

