Conducting clinical trials during the COVID-19 pandemic-a collaborative trial network response
- PMID: 33853661
- PMCID: PMC8045567
- DOI: 10.1186/s13063-021-05200-0
Conducting clinical trials during the COVID-19 pandemic-a collaborative trial network response
Abstract
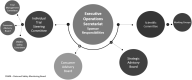
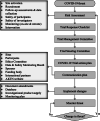
The unprecedented demand placed on healthcare systems from the COVID-19 pandemic has forced a reassessment of clinical trial conduct and feasibility. Consequently, the Australasian Kidney Trials Network (AKTN), an established collaborative research group known for conducting investigator-initiated global clinical trials, had to efficiently respond and adapt to the changing landscape during COVID-19. Key priorities included ensuring patient and staff safety, trial integrity and network sustainability for the kidney care community. New resources have been developed to enable a structured review and contingency plan of trial activities during the pandemic and beyond.
Conflict of interest statement
All authors have completed the Unified Competing Interest form (available on request from the corresponding author). YC has received research grants and speaker’s honoraria from Baxter Healthcare, Fresenius medical care and Amgen. She is a current recipient of the NHMRC Early Career Fellowship. AVi is a current recipient of a Jacquot Research Establishment Fellowship. DJ has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from Astra Zeneca and AWAK; speaker’s honoraria and travel sponsorships from ONO; and travel sponsorships from Amgen. He is a current recipient of an Australian National Health and Medical Research Council Practitioner Fellowship. CH has received grants from National Health and Medical Research Council, Shire and Baxter, and consultancy fees from GlaxoSmithKline and Astra Zeneca paid to her institution and outside the submitted work. MF has received research grants from Baxter Healthcare, Siemens Healthcare and Roche Diagnostics. NB has received an educational grant, advisory board consulting fees and honoraria from Baxter; advisory board consulting fees from Astra Zeneca; an educational grant and travel grant from Roche Pharmaceuticals; and a travel grant and educational grant from Amgen. RK has received consultancy fees, research grants, travel and speaker’s honoraria from Baxter Healthcare, travel sponsorships from Amgen and speaker’s honoraria from Shire. She is a current recipient of Queensland Advancing Clinical Research Fellowship. Staff members of AKTN are supported by NHMRC funding. The remaining authors have no competing interests to declare.
Figures


References
-
- Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, Collart F, Hemmelder MH, Ambühl P, Kerschbaum J, Legeai C, del Pino y Pino MD, Mircescu G, Mazzoleni L, Hoekstra T, Winzeler R, Mayer G, Stel VS, Wanner C, Zoccali C, Massy ZA. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. - DOI - PMC - PubMed
-
- Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, Thaunat O, Legris T, Moal V, Westeel PF, Kamar N, Gatault P, Snanoudj R, Sicard A, Bertrand D, Colosio C, Couzi L, Chemouny JM, Masset C, Blancho G, Bamoulid J, Duveau A, Bouvier N, Chavarot N, Grimbert P, Moulin B, le Meur Y, Hazzan M, Caillard S, Moulin B, Fafi-Kremer S, Hazzan M, Anglicheau D, Hertig A, Tourret J, Barrou B, Morelon E, Thaunat O, Couzi L, Merville P, Moal V, Legris T, Westeel PF, Jaureguy M, Frimat L, Ducloux D, Bamoulid J, Bertrand D, Tsimaratos M, Garaix-Gilardo F, Dumortier J, Mussot S, Roux A, Sebbag L, le Meur Y, Blancho G, Masset C, Kamar N, Francois H, Rondeau E, Bouvier N, Mousson C, Buchler M, Gatault P, Augusto JF, Duveau A, Vigneau C, Morin MC, Chemouny J, Golbin L, Grimbert P, Matignon M, Durrbach A, Greze C, Snanoudj R, Colosio C, Schvartz B, Malvezzi P, Mariat C, Thierry A, le Quintrec M, Sicard A, Rerolle JP, Heng AÉ, Garrouste C, Coponat HV, Epailly É, Brugiere O, Dharancy S, Salame É, Saliba F. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. - DOI - PMC - PubMed
-
- Tong A, Crowe S, Chando S, Cass A, Chadban SJ, Chapman JR, Gallagher M, Hawley CM, Hill S, Howard K, Johnson DW, Kerr PG, McKenzie A, Parker D, Perkovic V, Polkinghorne KR, Pollock C, Strippoli GFM, Tugwell P, Walker RG, Webster AC, Wong G, Craig JC. Research priorities in CKD: report of a national workshop conducted in Australia. Am J Kidney Dis. 2015;66(2):212–222. doi: 10.1053/j.ajkd.2015.02.341. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

