Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB
- PMID: 33853677
- PMCID: PMC8048169
- DOI: 10.1186/s13287-021-02313-w
Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB
Abstract
Background: Human dental follicle cells (DFCs) are the precursor cells of the periodontium with a high potential for regenerative therapies of (alveolar) bone. However, the molecular mechanisms of osteogenic differentiation are inadequately understood. Classical isoforms of protein kinase C (PKC) are reported to inhibit osteogenesis of stem/precursor cells. This study evaluated the role of classical PKCs and potential downstream targets on the osteogenic differentiation of DFCs.
Methods: DFCs were osteogenic differentiated with dexamethasone or bone morphogenetic protein 2 (BMP2). Expression of PKC and potential upstream/downstream regulators was manipulated using activators, inhibitors, and small interfering ribonucleic acid (siRNA). Expression of proteins was examined by Western blot analysis, while the activation levels of enzymes and transcription factors were examined by their phosphorylation states or by specific activation assays. Expression levels of osteogenic markers were examined by RT-qPCR (reverse transcription-quantitative polymerase chain reaction) analysis. Activity of alkaline phosphatase (ALP) and accumulation of calcium nodules by Alizarin Red staining were measured as indicators of mineralization.
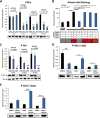
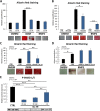
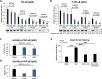
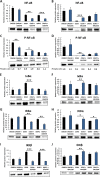
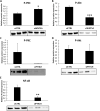
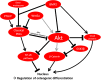
Results: Classical PKCs like PKCα inhibit the osteogenic differentiation of DFCs, but do not interfere with the induction of differentiation. Inhibition of classical PKCs by Gö6976 enhanced activity of Akt after osteogenic induction. Akt was also regulated during differentiation and especially disturbed BMP2-induced mineralization. The PKC/Akt axis was further shown to regulate the canonical Wnt signaling pathway and eventually nuclear expression of active β-catenin during dexamethasone-induced osteogenesis. Moreover, the nuclear factor "kappa-light-chain-enhancer" of activated B cells (NF-κB) pathway is regulated during osteogenic differentiation of DFCs and via the PKC/Akt axis and disturbs the mineralization. Upstream, parathyroid hormone-related protein (PTHrP) sustained the activity of PKC, while Wnt5a inhibited it.
Conclusions: Our results demonstrate that classical PKCs like PKCα and Akt regulate the osteogenic differentiation of DFCs partly via both β-catenin and NF-κB.
Keywords: Akt; Canonical Wnt signaling; Dental follicle cells; Mineralization; NF-κB; Osteogenic differentiation; Protein kinase C; β-catenin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells.Int J Mol Sci. 2022 May 25;23(11):5945. doi: 10.3390/ijms23115945. Int J Mol Sci. 2022. PMID: 35682637 Free PMC article. Review.
-
Evaluation of Current Studies to Elucidate Processes in Dental Follicle Cells Driving Osteogenic Differentiation.Biomedicines. 2023 Oct 13;11(10):2787. doi: 10.3390/biomedicines11102787. Biomedicines. 2023. PMID: 37893160 Free PMC article. Review.
-
A protein kinase A (PKA)/β-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs).Cell Signal. 2015 Mar;27(3):598-605. doi: 10.1016/j.cellsig.2014.12.008. Epub 2014 Dec 19. Cell Signal. 2015. PMID: 25530217
-
The parathyroid hormone-related protein is secreted during the osteogenic differentiation of human dental follicle cells and inhibits the alkaline phosphatase activity and the expression of DLX3.Tissue Cell. 2016 Aug;48(4):334-9. doi: 10.1016/j.tice.2016.05.007. Epub 2016 Jun 1. Tissue Cell. 2016. PMID: 27368119
-
AMP-activated protein kinase and the down-stream activated process of autophagy regulate the osteogenic differentiation of human dental follicle cells.Arch Oral Biol. 2021 Feb;122:104951. doi: 10.1016/j.archoralbio.2020.104951. Epub 2020 Nov 12. Arch Oral Biol. 2021. PMID: 33254047
Cited by
-
Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells.Int J Mol Sci. 2022 May 25;23(11):5945. doi: 10.3390/ijms23115945. Int J Mol Sci. 2022. PMID: 35682637 Free PMC article. Review.
-
[Research Progress in the Molecular Regulatory Mechanisms of Alveolar Bone Restoration].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 Jan 20;55(1):31-38. doi: 10.12182/20240160501. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38322519 Free PMC article. Chinese.
-
Energy Metabolism and Lipidome Are Highly Regulated during Osteogenic Differentiation of Dental Follicle Cells.Stem Cells Int. 2022 Jul 16;2022:3674931. doi: 10.1155/2022/3674931. eCollection 2022. Stem Cells Int. 2022. PMID: 35903407 Free PMC article.
-
The Secretome of the Inductive Tooth Germ Exhibits Signals Required for Tooth Development.Bioengineering (Basel). 2025 Jan 21;12(2):96. doi: 10.3390/bioengineering12020096. Bioengineering (Basel). 2025. PMID: 40001617 Free PMC article.
-
Evaluation of Current Studies to Elucidate Processes in Dental Follicle Cells Driving Osteogenic Differentiation.Biomedicines. 2023 Oct 13;11(10):2787. doi: 10.3390/biomedicines11102787. Biomedicines. 2023. PMID: 37893160 Free PMC article. Review.
References
-
- Zavan B, Bressan E. Dental stem cells: regenerative potential. Basel: Springer International Publishing; 2016.
-
- Ullah I, Subbarao RB, Kim E-J, Bharti D, Jang S-J, Park J-S, Shivakumar SB, Lee SL, Kang D, Byun JH, Park BW, Rho GJ. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci. 2016;154:39–51. doi: 10.1016/j.lfs.2016.04.026. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

