Life Cycle Dominates the Volatilome Character of Dimorphic Fungus Coccidioides spp
- PMID: 33853870
- PMCID: PMC8546678
- DOI: 10.1128/mSphere.00040-21
Life Cycle Dominates the Volatilome Character of Dimorphic Fungus Coccidioides spp
Abstract
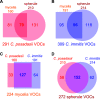
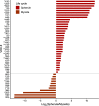
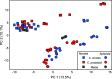
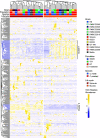
Valley fever (coccidioidomycosis) is an endemic fungal pneumonia of the North and South American deserts. The causative agents of Valley fever are the dimorphic fungi Coccidioides immitis and C. posadasii, which grow as mycelia in the environment and as spherules within the lungs of vulnerable hosts. Current diagnostics for Valley fever are severely lacking due to poor sensitivity and invasiveness, contributing to a 23-day median time to diagnosis, and therefore, new diagnostic tools are needed. We are working toward the development of a breath-based diagnostic for coccidioidomycosis, and in this initial study, we characterized the volatile metabolomes (or volatilomes) of in vitro cultures of Coccidioides Using solid-phase microextraction (SPME) and comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry (GC×GC-TOFMS), we characterized the volatile organic compounds (VOCs) produced by six strains of each species during mycelial or spherule growth. We detected a total of 353 VOCs that were at least 2-fold more abundant in a Coccidioides culture than in medium controls and found that the volatile metabolome of Coccidioides is more dependent on the growth phase (spherules versus mycelia) than on the species. The volatile profiles of C. immitis and C. posadasii have strong similarities, indicating that a single suite of Valley fever breath biomarkers can be developed to detect both species.IMPORTANCE Coccidioidomycosis, or Valley fever, causes up to 30% of community-acquired pneumonias in highly populated areas of the U.S. desert southwest where the disease is endemic. The infection is difficult to diagnose by standard serological and histopathological methods, which delays appropriate treatment. Therefore, we are working toward the development of breath-based diagnostics for Valley fever. In this study, we characterized the volatile metabolomes (or volatilomes) of six strains each of Coccidioides immitis and C. posadasii, the dimorphic fungal species that cause Valley fever. By analyzing the volatilomes during the two modes of growth of the fungus-mycelia and spherules-we observed that the life cycle plays a significant role in the volatiles produced by Coccidioides In contrast, we observed no significant differences in the C. immitis versus C. posadasii volatilomes. These data suggest that life cycle, rather than species, should guide the selection of putative biomarkers for a Valley fever breath test.
Keywords: GC×GC; Valley fever; biomarkers; coccidioidomycosis; comprehensive two-dimensional gas chromatography; dimorphic fungus; fungal infections; untargeted metabolomics; volatile metabolites.
Copyright © 2021 Higgins Keppler et al.
Figures

References
-
- Chiller T. 2019. Overview of endemic mycoses. NIAID, Rockville, MD.
-
- Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26:505–525. doi: 10.1128/CMR.00005-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
