Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin
- PMID: 33853873
- PMCID: PMC8546705
- DOI: 10.1128/mSphere.00250-21
Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin
Abstract
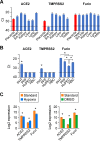
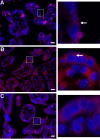
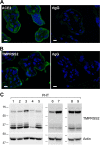
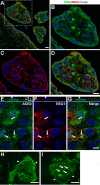
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a massive impact on human lives worldwide. While the airborne SARS-CoV-2 primarily affects the lungs, viremia is not uncommon. As placental trophoblasts are directly bathed in maternal blood, they are vulnerable to SARS-CoV-2. Intriguingly, the human fetus is largely spared from SARS-CoV-2 infection. We tested whether the human placenta expresses the main SARS-CoV-2 entry factors angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine 2 (TMPRSS2), and furin and showed that ACE2 and TMPRSS2 are expressed in the trophoblast rather than in other placental villous cells. While furin is expressed in the main placental villous cell types, we surveyed, trophoblasts exhibit the highest expression. In line with the expression of these entry factors, we demonstrated that a SARS-CoV-2 pseudovirus could enter primary human trophoblasts. Mechanisms underlying placental defense against SARS-CoV-2 infection likely involve postentry processing, which may be germane for mitigating interventions against SARS-CoV-2.IMPORTANCE Pregnant women worldwide have been affected by COVID-19. As the virus is commonly spread to various organs via the bloodstream and because human placental trophoblasts are directly bathed in maternal blood, feto-placental infection by SARS-CoV-2 seems likely. However, despite the heightened risk to pregnant women, thus far the transmission risk of COVID-19 to the feto-placental unit seems extremely low. This has been recently attributed to a negligible expression of SARS-CoV-2 entry factors in the human placenta. We therefore sought to explore the expression of the entry factors ACE2 and TMPRSS2 in the different cell types of human placental villi. Using a combination of transcriptome sequencing (RNA-seq), real-time quantitative PCR (RT-qPCR), in situ hybridization, and immunofluorescence, we found that trophoblasts, but not the other main villous cell types, express ACE2 and TMPRSS2, with a broad expression of furin. Correspondingly, we also showed that primary human trophoblasts are permissive to entry of SARS-CoV-2 pseudovirus particles.
Keywords: ACE2; SARS-CoV-2; TMPRSS2; furin; placenta; trophoblast.
Copyright © 2021 Ouyang et al.
Figures
References
-
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, HCA Lung Biological Network . 2020. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26:681–687. doi: 10.1038/s41591-020-0868-6. - DOI - PMC - PubMed
-
- Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. 2020. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium Position Paper. Front Immunol 11:1648. doi: 10.3389/fimmu.2020.01648. - DOI - PMC - PubMed
-
- Valdés G, Neves LA, Anton L, Corthorn J, Chacón C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J, Brosnihan KB. 2006. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 27:200–207. doi: 10.1016/j.placenta.2005.02.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
