Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia
- PMID: 33853929
- PMCID: PMC7610758
- DOI: 10.1126/scitranslmed.abc3961
Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia
Abstract
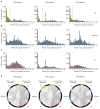
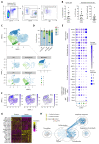
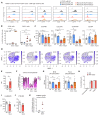
Rituximab (RTX), an antibody targeting CD20, is widely used as a first-line therapeutic strategy in B cell-mediated autoimmune diseases. However, a large proportion of patients either do not respond to the treatment or relapse during B cell reconstitution. Here, we characterize the cellular basis responsible for disease relapse in secondary lymphoid organs in humans, taking advantage of the opportunity offered by therapeutic splenectomy in patients with relapsing immune thrombocytopenia. By analyzing the B and plasma cell immunoglobulin gene repertoire at bulk and antigen-specific single-cell level, we demonstrate that relapses are associated with two responses coexisting in germinal centers and involving preexisting mutated memory B cells that survived RTX treatment and naive B cells generated upon reconstitution of the B cell compartment. To identify distinctive characteristics of the memory B cells that escaped RTX-mediated depletion, we analyzed RTX refractory patients who did not respond to treatment at the time of B cell depletion. We identified, by single-cell RNA sequencing (scRNA-seq) analysis, a population of quiescent splenic memory B cells that present a unique, yet reversible, RTX-shaped phenotype characterized by down-modulation of B cell-specific factors and expression of prosurvival genes. Our results clearly demonstrate that these RTX-resistant autoreactive memory B cells reactivate as RTX is cleared and give rise to plasma cells and further germinal center reactions. Their continued surface expression of CD19 makes them efficient targets for current anti-CD19 therapies. This study thus identifies a pathogenic contributor to autoimmune diseases that can be targeted by available therapeutic agents.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Dörner T, Lipsky PE. B cells: Depletion or functional modulation in rheumatic diseases. Curr Opin Rheumatol. 2014;26:228–236. - PubMed
-
- Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard J-F, Cheze S, Graveleau J, Slama B, Audia S, Ebbo M, Le Guenno G, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: Results from a prospective registry including 248 patients. Blood. 2014;124:3228–3236. - PubMed
-
- Lioger B, Edupuganti SR, Mulleman D, Passot C, Desvignes C, Bejan-Angoulvant T, Thibault G, Gouilleux-Gruart V, Mélet J, Paintaud G, Ternant D. Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients. Br J Clin Pharmacol. 2017;83:1773–1781. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

