Epidemiology and Molecular Basis of Multidrug Resistance in Rhodococcus equi
- PMID: 33853933
- PMCID: PMC8139527
- DOI: 10.1128/MMBR.00011-21
Epidemiology and Molecular Basis of Multidrug Resistance in Rhodococcus equi
Abstract
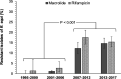
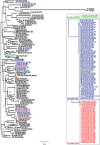
The development and spread of antimicrobial resistance are major concerns for human and animal health. The effects of the overuse of antimicrobials in domestic animals on the dissemination of resistant microbes to humans and the environment are of concern worldwide. Rhodococcus equi is an ideal model to illustrate the spread of antimicrobial resistance at the animal-human-environment interface because it is a natural soil saprophyte that is an intracellular zoonotic pathogen that produces severe bronchopneumonia in many animal species and humans. Globally, R. equi is most often recognized as causing severe pneumonia in foals that results in animal suffering and increased production costs for the many horse-breeding farms where the disease occurs. Because highly effective preventive measures for R. equi are lacking, thoracic ultrasonographic screening and antimicrobial chemotherapy of subclinically affected foals have been used for controlling this disease during the last 20 years. The resultant increase in antimicrobial use attributable to this "screen-and-treat" approach at farms where the disease is endemic has likely driven the emergence of multidrug-resistant (MDR) R. equi in foals and their environment. This review summarizes the factors that contributed to the development and spread of MDR R. equi, the molecular epidemiology of the emergence of MDR R. equi, the repercussions of MDR R. equi for veterinary and human medicine, and measures that might mitigate antimicrobial resistance at horse-breeding farms, such as alternative treatments to traditional antibiotics. Knowledge of the emergence and spread of MDR R. equi is of broad importance for understanding how antimicrobial use in domestic animals can impact the health of animals, their environment, and human beings.
Keywords: Rhodococcus equi; antibiotic resistance; antimicrobial agents; environmental microbiology; horizontal gene transfer; microbial genetics; zoonotic infections.
Copyright © 2021 American Society for Microbiology.
Figures



References
-
- Fernando DM, Tun HM, Poole J, Patidar R, Li R, Mi R, Amarawansha GEA, Fernando WGD, Khafipour E, Farenhorst A, Kumar A. 2016. Detection of antibiotic resistance genes in source and drinking water samples from a First Nations community in Canada. Appl Environ Microbiol 82:4767–4775. 10.1128/AEM.00798-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

