Perinatal Fentanyl Exposure Leads to Long-Lasting Impairments in Somatosensory Circuit Function and Behavior
- PMID: 33853934
- PMCID: PMC8051687
- DOI: 10.1523/JNEUROSCI.2470-20.2020
Perinatal Fentanyl Exposure Leads to Long-Lasting Impairments in Somatosensory Circuit Function and Behavior
Abstract
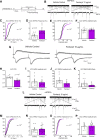
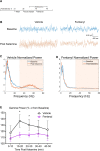
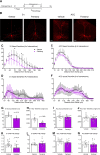
One consequence of the opioid epidemic are lasting neurodevelopmental sequelae afflicting adolescents exposed to opioids in the womb. A translationally relevant and developmentally accurate preclinical model is needed to understand the behavioral, circuit, network, and molecular abnormalities resulting from this exposure. By employing a novel preclinical model of perinatal fentanyl exposure, our data reveal that fentanyl has several dose-dependent, developmental consequences to somatosensory function and behavior. Newborn male and female mice exhibit signs of withdrawal and sensory-related deficits that extend at least to adolescence. As fentanyl exposure does not affect dams' health or maternal behavior, these effects result from the direct actions of perinatal fentanyl on the pups' developing brain. At adolescence, exposed mice exhibit reduced adaptation to sensory stimuli, and a corresponding impairment in primary somatosensory (S1) function. In vitro electrophysiology demonstrates a long-lasting reduction in S1 synaptic excitation, evidenced by decreases in release probability, NMDA receptor-mediated postsynaptic currents, and frequency of miniature excitatory postsynaptic currents (mEPSCs), as well as increased frequency of miniature inhibitory postsynaptic currents (mIPSCs). In contrast, anterior cingulate cortical neurons exhibit an opposite phenotype, with increased synaptic excitation. Consistent with these changes, electrocorticograms (ECoGs) reveal suppressed ketamine-evoked γ oscillations. Morphologic analysis of S1 pyramidal neurons indicate reduced dendritic complexity, dendritic length, and soma size. Further, exposed mice exhibited abnormal cortical mRNA expression of key receptors involved in synaptic transmission and neuronal growth and development, changes that were consistent with the electrophysiological and morphologic changes. These findings demonstrate the lasting sequelae of perinatal fentanyl exposure on sensory processing and function.SIGNIFICANCE STATEMENT This is the first study to show that exposure to fentanyl in the womb results in behavioral, circuitry, and synaptic effects that last at least to adolescence. We also show, for the first time, that this exposure has different, lasting effects on synapses in different cortical areas.
Keywords: Developmental Biology; opioid; somatosensory; thalamocortical.
Copyright © 2021 the authors.
Figures



References
-
- Alipio JB, Brockett AT, Fox ME, Tennyson SS, deBettencourt CA, El-Metwally D, Francis NA, Kanold PO, Lobo MK, Roesch MR, Keller A (2020) Enduring consequences of perinatal fentanyl exposure in mice. Addict Biol. Advance online publication. Retrieved March 18, 2020. doi: 10.1111/adb.12895. - DOI - PMC - PubMed
-
- Ayres AJ (1964) Tactile functions. Their relation to hyperactive and perceptual motor behavior. Am J Occup Ther 18:6–11. - PubMed
-
- Bakhireva LN, Holbrook BD, Shrestha S, Leyva Y, Ashley M, Cano S, Lowe J, Stephen JM, Leeman L (2019) Association between prenatal opioid exposure, neonatal opioid withdrawal syndrome, and neurodevelopmental and behavioral outcomes at 5-8 months of age. Early Hum Dev 128:69–76. 10.1016/j.earlhumdev.2018.10.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
