Sirt1 deacetylates and stabilizes p62 to promote hepato-carcinogenesis
- PMID: 33854041
- PMCID: PMC8046979
- DOI: 10.1038/s41419-021-03666-z
Sirt1 deacetylates and stabilizes p62 to promote hepato-carcinogenesis
Abstract
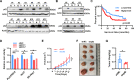
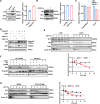
p62/SQSTM1 is frequently up-regulated in many cancers including hepatocellular carcinoma. Highly expressed p62 promotes hepato-carcinogenesis by activating many signaling pathways including Nrf2, mTORC1, and NFκB signaling. However, the underlying mechanism for p62 up-regulation in hepatocellular carcinoma remains largely unclear. Herein, we confirmed that p62 was up-regulated in hepatocellular carcinoma and its higher expression was associated with shorter overall survival in patients. The knockdown of p62 in hepatocellular carcinoma cells decreased cell growth in vitro and in vivo. Intriguingly, p62 protein stability could be reduced by its acetylation at lysine 295, which was regulated by deacetylase Sirt1 and acetyltransferase GCN5. Acetylated p62 increased its association with the E3 ligase Keap1, which facilitated its poly-ubiquitination-dependent proteasomal degradation. Moreover, Sirt1 was up-regulated to deacetylate and stabilize p62 in hepatocellular carcinoma. Additionally, Hepatocyte Sirt1 conditional knockout mice developed much fewer liver tumors after Diethynitrosamine treatment, which could be reversed by the re-introduction of exogenous p62. Taken together, Sirt1 deacetylates p62 at lysine 295 to disturb Keap1-mediated p62 poly-ubiquitination, thus up-regulating p62 expression to promote hepato-carcinogenesis. Therefore, targeting Sirt1 or p62 is a reasonable strategy for the treatment of hepatocellular carcinoma.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
The Ubiquitin E3 Ligase TRIM21 Promotes Hepatocarcinogenesis by Suppressing the p62-Keap1-Nrf2 Antioxidant Pathway.Cell Mol Gastroenterol Hepatol. 2021;11(5):1369-1385. doi: 10.1016/j.jcmgh.2021.01.007. Epub 2021 Jan 19. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33482392 Free PMC article.
-
Negative Regulation of the Keap1-Nrf2 Pathway by a p62/Sqstm1 Splicing Variant.Mol Cell Biol. 2018 Mar 15;38(7):e00642-17. doi: 10.1128/MCB.00642-17. Print 2018 Apr 1. Mol Cell Biol. 2018. PMID: 29339380 Free PMC article.
-
SQSTM1/p62 Knockout by Using the CRISPR/Cas9 System Inhibits Migration and Invasion of Hepatocellular Carcinoma.Cells. 2023 Apr 25;12(9):1238. doi: 10.3390/cells12091238. Cells. 2023. PMID: 37174639 Free PMC article.
-
Sequestosome 1/p62-related pathways as therapeutic targets in hepatocellular carcinoma.Expert Opin Ther Targets. 2019 May;23(5):393-406. doi: 10.1080/14728222.2019.1601703. Epub 2019 Apr 15. Expert Opin Ther Targets. 2019. PMID: 30987486 Review.
-
Emerging role of silent information regulator 1 (SIRT1) in hepatocellular carcinoma: a potential therapeutic target.Tumour Biol. 2015 Jun;36(6):4063-74. doi: 10.1007/s13277-015-3488-x. Epub 2015 May 1. Tumour Biol. 2015. PMID: 25926383 Review.
Cited by
-
The Role of Sirtuin-1 (SIRT1) in the Physiology and Pathophysiology of the Human Placenta.Int J Mol Sci. 2023 Nov 11;24(22):16210. doi: 10.3390/ijms242216210. Int J Mol Sci. 2023. PMID: 38003402 Free PMC article. Review.
-
Core-predominant gut fungus Kazachstania slooffiae promotes intestinal epithelial glycolysis via lysine desuccinylation in pigs.Microbiome. 2023 Feb 23;11(1):31. doi: 10.1186/s40168-023-01468-3. Microbiome. 2023. PMID: 36814349 Free PMC article.
-
Deciphering a profiling based on multiple post-translational modifications functionally associated regulatory patterns and therapeutic opportunities in human hepatocellular carcinoma.Mol Cancer. 2024 Dec 28;23(1):283. doi: 10.1186/s12943-024-02199-1. Mol Cancer. 2024. PMID: 39732660 Free PMC article.
-
p62/SQSTM1 in cancer: phenomena, mechanisms, and regulation in DNA damage repair.Cancer Metastasis Rev. 2025 Feb 15;44(1):33. doi: 10.1007/s10555-025-10250-w. Cancer Metastasis Rev. 2025. PMID: 39954143 Free PMC article. Review.
-
NS1 binding protein regulates stress granule dynamics and clearance by inhibiting p62 ubiquitination.Nat Commun. 2024 Dec 30;15(1):10925. doi: 10.1038/s41467-024-55446-w. Nat Commun. 2024. PMID: 39738171 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

