Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1
- PMID: 33854057
- PMCID: PMC8046803
- DOI: 10.1038/s41467-021-22471-y
Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1
Abstract
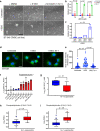
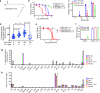
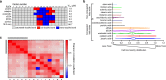
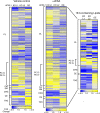
Ferroptosis is associated with lipid hydroperoxides generated by the oxidation of polyunsaturated acyl chains. Lipid hydroperoxides are reduced by glutathione peroxidase 4 (GPX4) and GPX4 inhibitors induce ferroptosis. However, the therapeutic potential of triggering ferroptosis in cancer cells with polyunsaturated fatty acids is unknown. Here, we identify conjugated linoleates including α-eleostearic acid (αESA) as ferroptosis inducers. αESA does not alter GPX4 activity but is incorporated into cellular lipids and promotes lipid peroxidation and cell death in diverse cancer cell types. αESA-triggered death is mediated by acyl-CoA synthetase long-chain isoform 1, which promotes αESA incorporation into neutral lipids including triacylglycerols. Interfering with triacylglycerol biosynthesis suppresses ferroptosis triggered by αESA but not by GPX4 inhibition. Oral administration of tung oil, naturally rich in αESA, to mice limits tumor growth and metastasis with transcriptional changes consistent with ferroptosis. Overall, these findings illuminate a potential approach to ferroptosis, complementary to GPX4 inhibition.
Conflict of interest statement
Ulrike Rennefahrt is a former employee of Metanomics Health, GmbH. Marcus Conrad is an inventor on patents for some of the compounds described herein, and shareholder of ROSCUE Therapeutics GmbH. The remaining authors declare no competing interests.
Figures


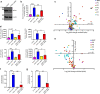
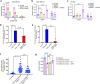
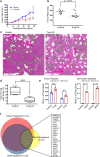
References
-
- Friedmann-Angeli, J. P., Miyamoto, S. & Schulze, A. Ferroptosis: the greasy side of cell death. Chem. Res. Toxicol.32, 362–369 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

