Circ-OSBPL2 Contributes to Smoke-Related Chronic Obstructive Pulmonary Disease by Targeting miR-193a-5p/BRD4 Axis
- PMID: 33854310
- PMCID: PMC8039023
- DOI: 10.2147/COPD.S298465
Circ-OSBPL2 Contributes to Smoke-Related Chronic Obstructive Pulmonary Disease by Targeting miR-193a-5p/BRD4 Axis
Abstract
Background: Circular RNAs (circRNAs) have been identified to play roles in the respiratory diseases. Here, this study aimed to elucidate the function of circRNA oxysterol binding protein like 2 (circOSBPL2) in the development of smoke-related chronic obstructive pulmonary diseases (COPD).
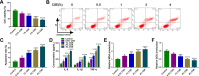
Methods: The expression of circ-OSBPL2, microRNA (miR)-193a-5p, and bromodomain-containing protein 4 (BRD4) was detected using qRT-PCR and Western blot assays. Cigarette smoke extract (CSE)-induced human bronchial epithelial cells (HBECs) was applied to mimic smoke-related COPD in vitro. Flow cytometric analysis of cell apoptosis and ELISA analysis of interleukins (IL)-6, IL-8, tumor necrosis factor-α (TNF-α) levels were performed. The malondialdehyde (MDA) and superoxide dismutase (SOD) production levels were analyzed according to the kit instructions. The binding interaction between miR-193a-5p and circ-OSBPL2 or BRD4 was confirmed by dual-luciferase reporter assay and RNA immunoprecipitation assays.
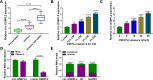
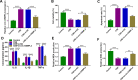
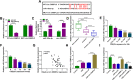
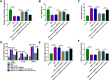
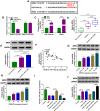
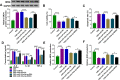
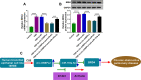
Results: Circ-OSBPL2 was highly expressed in lung tissues of smokers without or with COPD, particularly in smokers with COPD. Also, the expression of circ-OSBPL2 was dose and time-dependently elevated in CSE-induced HBECs. Circ-OSBPL2 down-regulation in HBECs attenuated CSE-evoked cell proliferation arrest, and cell apoptosis, inflammation and oxidative stress promotion. Mechanistically, circ-OSBPL2 served as a sponge for miR-193a-5p, and miR-193a-5p inhibition reversed the effects of circ-OSBPL2 knockdown on CSE-mediated HBECs. Besides that, miR-193a-5p directly targeted BRD4, and miR-193a-5p re-expression in HBECs abolished CSE-induced HBEC injury, which was reverted by BRD4 up-regulation. Additionally, we also found circ-OSBPL2 could indirectly regulate BRD4 via miR-193a-5p.
Conclusion: Circ-OSBPL2 contributed to the apoptosis, inflammation, and oxidative stress of HBECs in smoke-related COPD by miR-193a-5p/BRD4 axis, suggesting a novel insight on the pathogenesis of COPD and a potential therapeutic strategy for future clinic intervention in COPD.
Keywords: BRD4; COPD; circ-OSBPL2; miR-193a-5p.
© 2021 Zheng et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

