Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid-Polymer Hybrid Nanoparticles
- PMID: 33854311
- PMCID: PMC8039437
- DOI: 10.2147/IJN.S293480
Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid-Polymer Hybrid Nanoparticles
Abstract
Background: Plumbagin, a naphthoquinone extracted from the officinal leadwort presenting promising anti-cancer properties, has its therapeutic potential limited by its inability to reach tumors in a specific way at a therapeutic concentration following systemic injection. The purpose of this study is to assess whether a novel tumor-targeted, lipid-polymer hybrid nanoparticle formulation of plumbagin would suppress the growth of B16-F10 melanoma in vitro and in vivo.
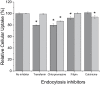
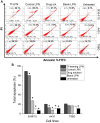
Methods: Novel lipid-polymer hybrid nanoparticles entrapping plumbagin and conjugated with transferrin, whose receptors are present in abundance on many cancer cells, have been developed. Their cellular uptake, anti-proliferative and apoptosis efficacy were assessed on various cancer cell lines in vitro. Their therapeutic efficacy was evaluated in vivo after tail vein injection to mice bearing B16-F10 melanoma tumors.
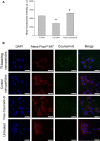
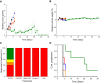
Results: The transferrin-bearing lipid-polymer hybrid nanoparticles loaded with plumbagin resulted in the disappearance of 40% of B16-F10 tumors and regression of 10% of the tumors following intravenous administration. They were well tolerated by the mice.
Conclusion: These therapeutic effects, therefore, make transferrin-bearing lipid-polymer hybrid nanoparticles entrapping plumbagin a highly promising anti-cancer nanomedicine.
Keywords: cancer therapy; lipid–polymer hybrid nanoparticles; plumbagin; transferrin; tumor targeting.
© 2021 Sakpakdeejaroen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Anti-Tumor Activity of Intravenously Administered Plumbagin Entrapped in Targeted Nanoparticles.J Biomed Nanotechnol. 2020 Jan 1;16(1):85-100. doi: 10.1166/jbn.2020.2874. J Biomed Nanotechnol. 2020. PMID: 31996288
-
Transferrin-bearing liposomes entrapping plumbagin for targeted cancer therapy.J Interdiscip Nanomed. 2019 Jun 26;4(2):54-71. doi: 10.1002/jin2.56. eCollection 2019 Jun. J Interdiscip Nanomed. 2019. PMID: 31341642 Free PMC article.
-
Tumor regression after intravenous administration of targeted vesicles entrapping the vitamin E α-tocotrienol.J Control Release. 2017 Jan 28;246:79-87. doi: 10.1016/j.jconrel.2016.12.014. Epub 2016 Dec 18. J Control Release. 2017. PMID: 27993600
-
Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy.Food Chem Toxicol. 2019 Mar;125:566-582. doi: 10.1016/j.fct.2019.01.018. Epub 2019 Jan 24. Food Chem Toxicol. 2019. PMID: 30685472 Review.
-
Anticancer Properties and Pharmaceutical Applications of Plumbagin: A Review.Am J Chin Med. 2017;45(3):423-441. doi: 10.1142/S0192415X17500264. Epub 2017 Mar 30. Am J Chin Med. 2017. PMID: 28359198 Review.
Cited by
-
Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review.Curr Oncol. 2023 Jul 25;30(8):7112-7131. doi: 10.3390/curroncol30080516. Curr Oncol. 2023. PMID: 37622997 Free PMC article.
-
Surface Functionalised Parenteral Nanoemulsions for Active and Homotypic Targeting to Melanoma.Pharmaceutics. 2023 Apr 28;15(5):1358. doi: 10.3390/pharmaceutics15051358. Pharmaceutics. 2023. PMID: 37242600 Free PMC article.
-
Report on Webinar Series Cell and Gene Therapy: From Concept to Clinical Use.Pharmaceutics. 2022 Jan 11;14(1):168. doi: 10.3390/pharmaceutics14010168. Pharmaceutics. 2022. PMID: 35057063 Free PMC article.
-
Highlights on Cell-Penetrating Peptides and Polymer-Lipid Hybrid Nanoparticle: Overview and Therapeutic Applications for Targeted Anticancer Therapy.AAPS PharmSciTech. 2023 May 24;24(5):124. doi: 10.1208/s12249-023-02576-x. AAPS PharmSciTech. 2023. PMID: 37225901 Review.
-
Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy.Cancers (Basel). 2022 May 17;14(10):2473. doi: 10.3390/cancers14102473. Cancers (Basel). 2022. PMID: 35626078 Free PMC article. Review.
References
-
- Hsu YL, Cho CY, Kuo PL, et al. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J Pharmacol Exp Ther. 2006;318:484–494. doi:10.1124/jpet.105.098863 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

