Severe, Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccine
- PMID: 33854395
- PMCID: PMC8040692
- DOI: 10.2147/JBM.S307047
Severe, Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccine
Abstract
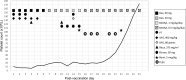
The rollout of the SARS-CoV-2 vaccine is underway, and millions have already been vaccinated. At least 25 reports of "immune thrombocytopenia" (ITP) or "thrombocytopenia" following the Moderna or Pfizer vaccine have been added to the Vaccine Adverse Event Reporting System (VAERS) in the US. ITP is a rare but known complication of several vaccinations. SARS-CoV-2 vaccine is new, with a novel mechanism of action, and understanding the epidemiology, clinical manifestations, treatment success and natural history of post-vaccination thrombocytopenia is evolving. We report a 74-year-old man who developed refractory thrombocytopenia within one day of receiving the Moderna SARS-CoV-2 vaccine. Several hours after vaccination, he developed significant epistaxis and cutaneous purpura. Severe thrombocytopenia was documented the following day, and he developed extremity weakness and encephalopathy with facial muscle weakness. Over a 14-day period, thrombocytopenia was treated first with high dose dexamethasone, intravenous immunoglobulin, platelet transfusions, rituximab, plasma exchange (for presumed acute inflammatory demyelinating polyneuropathy (AIDP)), and four daily doses of the thrombopoietin receptor agonist (TPO-RA) eltrombopag (Promacta™), without a platelet response. Three days later, he received the TPO-RA romiplostim (Nplate™). Five days later, his platelet count began to rise and by post-vaccination day 25, his platelet count was in the normal range. Thrombocytopenia was refractory to frontline and second-line treatment. The eventual rise in his platelet count suggests that one or both TPO-RAs may have impacted platelet recovery. Possibly, but less likely given the temporality, the drug-induced thrombocytopenia was subsiding. The aggressive use of immunosuppressive treatment may jeopardize the intended purpose of the SARS-CoV-2 vaccine, and earlier use of non-immunosuppressive second-line treatment for vaccine-related severe thrombocytopenia, such as with TPO-RAs, should be considered. While it is imperative to continue the global vaccination program, vigilance to the occurrence of post-vaccination severe thrombocytopenia is warranted.
Keywords: SARS-CoV-2; immune thrombocytopenic purpura; platelet; thrombocytopenia; thrombopoietin receptor agonist; vaccine.
© 2021 Helms et al.
Conflict of interest statement
Dr Michael D Tarantino reports personal fees for consulting and/or speaking from and past clinical trials investigator for Amgen and Dova, outside the submitted work. The authors report no other conflicts of interest in this work.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous