Cycling of tumor necrosis factor inhibitors versus switching to different mechanism of action therapy in rheumatoid arthritis patients with inadequate response to tumor necrosis factor inhibitors: a Bayesian network meta-analysis
- PMID: 33854570
- PMCID: PMC8010806
- DOI: 10.1177/1759720X211002682
Cycling of tumor necrosis factor inhibitors versus switching to different mechanism of action therapy in rheumatoid arthritis patients with inadequate response to tumor necrosis factor inhibitors: a Bayesian network meta-analysis
Abstract
Introduction: For patients with rheumatoid arthritis (RA) with an inadequate response to tumor necrosis factor inhibitors (TNFi), main options include cycling onto a different TNFi or switching to a biologic/targeted synthetic disease-modifying antirheumatic drug with a different mechanism of action (MOA). This network meta-analysis (NMA) assessed comparative clinical efficacy of cycling versus switching.
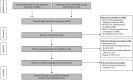
Methods: We conducted a literature search in MEDLINE, Embase, and Cochrane Library. Outcomes included proportion of patients with 20%, 50%, or 70% response to American College of Rheumatology criteria (ACR20/ACR50/ACR70 response), Disease Activity Score in 28 joints (DAS28) score below 2.6 or between 2.6 and 3.2, mean change in DAS28 score, mean reduction in and proportion of patients achieving a clinically meaningful reduction (⩾0.22) in Health Assessment Questionnaire score, number of serious adverse events (AEs), and withdrawals for any reason/due to AEs/lack of treatment efficacy. To account for the wide range of study populations and designs, we developed three models to conduct the NMA: fixed-effect, random-effects, and hierarchical Bayesian. PROSPERO ID: CRD42019122993.
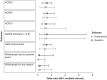
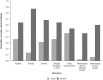
Results: We identified nine randomized controlled trials and 16 observational studies. The fixed-effect model suggested a 0.99 probability that switch was the better strategy for increasing odds of a clinically meaningful improvement in ACR50 [odds ratio (OR): 1.35 (95% credible interval (CI): 0.96-1.81)]. The fixed-effect model also suggested that switch was associated with lower rates of withdrawal for any reasons [OR: 0.53 (95% CI: 0.40-0.68)]. The random-effects and hierarchical Bayesian models suggested additional uncertainty as they considered more variability than the fixed-effect model.
Discussion: Results suggest that switching to a drug with a different MOA is more effective and associated with lower rates of withdrawal than cycling to a different TNFi after failure of first-line TNFi. Further trials that directly compare cycling with switching are warranted to better assess comparative efficacy.
Plain language summary: Assessment of the effectiveness of different drug treatment strategies in patients with rheumatoid arthritis: an analysis of the published literature Rheumatoid arthritis (RA) is a chronic disease in which inflammation affects joints along with the entire body; this may cause significant pain, joint damage, physical disability, a decreased quality of life, and an increased risk of death.Tumor necrosis factor inhibitors (TNFis) are a common choice as first-line drugs to treat RA. Although they are effective in many patients, therapy with a TNFi is not successful within the first year of treatment in approximately one-third of patients due to either a lack of efficacy or safety issues.When TNFi therapy is unsuccessful, the options are to "cycle" to another TNFi or to "switch" to another drug with a different mechanism of action (MOA). Further studies are needed to help doctors decide the best treatment strategy for their patients when treatment with an initial TNFi fails.This study analyzed 25 published studies in which patients were either "cycled" to another TNFi or "switched" to a drug with a different MOA after unsuccessful treatment with an initial TNFi.The results showed that "switching" to a drug with a different MOA was a better treatment strategy than "cycling" to another TNFi; "switching" increased the chance of clinically meaningful improvement in disease status and lowered the chance of having to stop treatment for any reason.
Keywords: disease-modifying antirheumatic drugs; network meta-analysis; rheumatoid arthritis; tumor necrosis factor.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: AM acted as a consultant for Bristol Myers Squibb, Fidia Pharma, IBSA, Merck, Pfizer, Roche, Sanofi-Aventis and UCB. GP is an employee of ISHEO SRL. DI received grant/research support from AbbVie, Angelini, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Fidia Pharma, Merck Serono and Pierre Fabre, and is an employee of ISHEO SRL. JZ is an employee of and shareholder in Bristol Myers Squibb. EA was an employee of and shareholder in Bristol Myers Squibb at the time of the analysis.
Figures
References
-
- Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet 2010; 376: 1094–1108. - PubMed
-
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. - PubMed
-
- Favalli EG, Raimondo MG, Becciolini A, et al. The management of first-line biologic therapy failures in rheumatoid arthritis: current practice and future perspectives. Autoimmun Rev 2017; 16: 1185–1195. - PubMed
-
- Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008; 67: 1516–1523. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

