Reduction of anterior uveitis flares in patients with axial spondyloarthritis on certolizumab pegol treatment: final 2-year results from the multicenter phase IV C-VIEW study
- PMID: 33854572
- PMCID: PMC8010825
- DOI: 10.1177/1759720X211003803
Reduction of anterior uveitis flares in patients with axial spondyloarthritis on certolizumab pegol treatment: final 2-year results from the multicenter phase IV C-VIEW study
Abstract
Introduction: Acute anterior uveitis (AAU), affecting up to 40% of patients with axial spondyloarthritis (axSpA), risks permanent visual deficits if not adequately treated. We report 2-year results from C-VIEW, the first study to prospectively investigate certolizumab pegol (CZP) on AAU in patients with active axSpA at high risk of recurrent AAU.
Patients and methods: C-VIEW (NCT03020992) was a 104-week (96 weeks plus 8-week safety follow-up), open-label, multicenter study. Eligible patients had active axSpA, human leukocyte antigen-B27 (HLA-B27) positivity and a history of recurrent AAU (⩾2 AAU flares in total; ⩾1 in the year prior to baseline). Patients received CZP 400 mg at weeks 0, 2 and 4, then 200 mg every 2 weeks to week 96. The primary efficacy endpoint was the AAU flare event rate during 96 weeks' CZP versus 2 years pre-baseline.
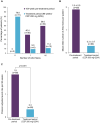
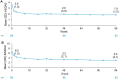
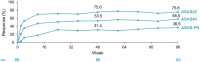
Results: Of 115 enrolled patients, 89 initiated CZP (male: 63%; radiographic/non-radiographic axSpA: 85%/15%; mean disease duration: 9.1 years); 83 completed week 96. There was a significant 82% reduction in AAU flare event rate during CZP versus pre-baseline [rate ratio (95% confidence interval): 0.18 (0.12-0.28), p < 0.001]. One hundred percent and 59.6% of patients experienced ⩾1 and ⩾2 AAU flares pre-baseline, respectively, compared to 20.2% and 11.2% during treatment. Age, sex and axSpA population subgroup analyses were consistent with the primary analysis. There were substantial improvements in axSpA disease activity with no new safety signal identified.
Conclusion: CZP treatment significantly reduced AAU flare event rate in patients with axSpA and a history of AAU, indicating CZP is a suitable treatment option for patients at risk of recurrent AAU.
Trial registration clinicaltrialsgov: NCT03020992, URL: https://clinicaltrials.gov/ct2/show/NCT03020992.
Keywords: TNF inhibitor; axial spondyloarthritis; extra-articular manifestations; uveitis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: Irene E. van der Horst-Bruinsma: Honoraria/consulting fees/research grants from AbbVie, BMS, MSD, Novartis, Pfizer, UCB Pharma; Rianne E. van Bentum: none; Frank D. Verbraak: Honoraria/consulting fees/research grants from Bayer, Novartis, IDxDR, UCB Pharma; Atul Deodhar: Honoraria/consulting fees/research grants from AbbVie, Amgen, Boehringer Ingelheim, BMS, Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB Pharma; Thomas Rath: Honoraria/consulting fees from AbbVie, BMS, Chugai, Eli Lilly, MSD, Novartis, Pfizer, Roche, UCB Pharma; Bengt Hoepken, Oscar Irvin-Sellers, Lars Bauer: Employees of UCB Pharma; Karen Thomas: Former contractor with UCB Pharma; Martin Rudwaleit: Honoraria/consulting fees from AbbVie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Pfizer, Roche, UCB Pharma.
Figures
References
-
- Bacchiega ABS, Balbi GGM, Ochtrop MLG, et al. Ocular involvement in patients with spondyloarthritis. Rheumatology (Oxford) 2017; 56: 2060–2067. - PubMed
-
- Rosenbaum JT, Smith JR. Anti-TNF therapy for eye involvement in spondyloarthropathy. Clin Exp Rheumatol 2002; 20: S143–S145. - PubMed
-
- Frantz C, Portier A, Etcheto A, et al. Acute anterior uveitis in spondyloarthritis: a monocentric study of 301 patients. Clin Exp Rheumatol 2019; 37: 26–31. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

