The 2019 European Union report on pesticide residues in food
- PMID: 33854575
- PMCID: PMC8025100
- DOI: 10.2903/j.efsa.2021.6491
The 2019 European Union report on pesticide residues in food
Abstract
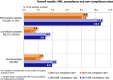
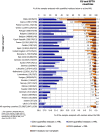
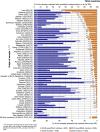
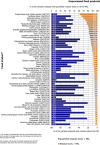
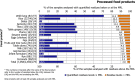
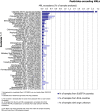
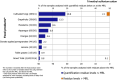
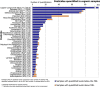
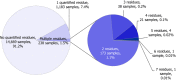
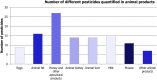
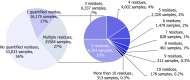
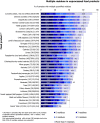
Under European Union legislation (Article 32, Regulation (EC) No 396/2005), the EFSA provides an annual report which examines pesticide residue levels in foods on the European market. This report is based on data from the official national control activities carried out by EU Member States, Iceland and Norway and includes a subset of data from the EU-coordinated control programme which uses a randomised sampling strategy. For 2019, 96.1% of the overall 96,302 samples analysed fell below the maximum residue level (MRL), 3.9% exceeded this level, of which 2.3% were non-compliant, i.e. samples exceeding the MRL after taking the measurement uncertainty into account. For the subset of 12,579 samples analysed as part of the EU-coordinated control programme, 2.0% exceeded the MRL and 1.0% were non-compliant. To assess acute and chronic risk to consumer health, dietary exposure to pesticide residues was estimated and compared with health-based guidance values. The findings suggest that the residue levels for the food commodities analysed are unlikely to pose any concern for consumer health. However, a number of recommendations are proposed to increase the effectiveness of European control systems, thereby continuing to ensure a high level of consumer protection throughout the EU.
Keywords: European Union; acute; chronic; dietary exposure; food safety; maximum residue levels; national monitoring programme; pesticide residues; risk assessment.
© 2021 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures





















References
-
- Codex (Codex Alimentarius Commission), 2006. CAG/GL 59‐2006 Guidelines on Estimation of Uncertainty of Results. Codex Alimentarius Commission, Rome Italy, 2006. Available online: www.codexalimentarius.net/download/report/701/al31_24e.pdf
-
- EFSA (European Food Safety Authority), 2011. Review of the existing maximum residue levels (MRLs) for tebuconazole according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(8):2339, 96 pp. 10.2903/j.efsa.2011.2339 - DOI
-
- EFSA (European Food Safety Authority), 2015b. Reasoned opinion on the review of the existing maximum residue levels for deltamethrin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2015;13(11):4309, 104 pp. 10.2903/j.efsa.2015.4309 - DOI
-
- EFSA (European Food Safety Authority), 2015c. Reasoned opinion on the revision of the review of the existing maximum residue levels for lambda‐cyhalothrin. EFSA Journal 2015;13(12):4324, 119 pp. 10.2903/j.efsa.2015.4324 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
