Acemannan-induced tooth socket healing: A 12-month randomized controlled trial
- PMID: 33854714
- PMCID: PMC8025196
- DOI: 10.1016/j.jds.2020.10.003
Acemannan-induced tooth socket healing: A 12-month randomized controlled trial
Abstract
Background/purpose: Natural compounds have become alternatives for bone regeneration. Acemannan, the main polysaccharide extracted from Aloe vera, has been demonstrated as a promising osteoinductive material in vitro and in vivo. This clinical study investigated the effect of acemannan on tooth socket healing.
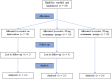
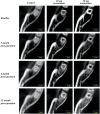
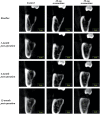
Materials and methods: Thirty-five otherwise healthy patients, 18-25 years old and diagnosed with horizontal or vertical partial impaction of the lower third molars, were enrolled in this randomized controlled trial. After removing the teeth, the sockets randomly received one of the following treatments: spontaneous blood-clotting (control), 20 mg acemannan sponge, or 50 mg acemannan sponge. Cone-beam computed tomography of the mandible was performed immediately (baseline), and at 3-, 6-, and 12-months postoperatively; the data were analyzed using the OsiriX MD program. Bone healing in the socket was determined measuring the socket volume. One-way ANOVA was used to analyze the differences within each group and between groups.
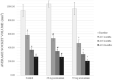
Results: Thirty-five patients with 43 partially impacted lower third molars participated in this study. No patients exhibited alveolar osteitis or secondary infection. Compared with baseline, all groups showed significant reduction in socket volume at all observation time-points (p < 0.05). The 50 mg acemannan group had a significantly greater reduction in socket volume compared with the control at all postoperative time-points (p < 0.05). The 20 mg group had a significantly greater reduction in socket volume compared with the control at 3-months postoperatively (p < 0.05).
Conclusion: We conclude that acemannan increases bone healing at 3-, 6-, and 12-months after removal of partially impacted mandibular third molars.
Keywords: Acemannan; Biomaterial; CBCT; Clinical study; Tooth socket healing.
© 2020 Association for Dental Sciences of the Republic of China. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Figures


References
-
- Kugelberg C.F. Impacted lower third molars and periodontal health. an epidemiological, methodological, retrospective and prospective clinical, study. Swed Dent J Suppl. 1990;68:1–52. - PubMed
-
- Bartee B.K. Extraction site reconstruction for alveolar ridge preservation. part 1: rationale and materials selection. J Oral Implantol. 2001;27:187–193. - PubMed
-
- Molly L., Vandromme H., Quirynen M., Schepers E., Adams J.L., van Steenberghe D. Bone formation following implantation of bone biomaterials into extraction sites. J Periodontol. 2008;79:1108–1115. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

