Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies
- PMID: 33854820
- PMCID: PMC8018489
- DOI: 10.1080/2162402X.2021.1900508
Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies
Abstract
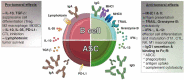
Tertiary lymphoid structures (TLS) are ectopic cellular aggregates that resemble secondary lymphoid organs in their composition and structural organization. In contrast to secondary lymphoid organs, TLS are not imprinted during embryogenesis but are formed in non-lymphoid tissues in response to local inflammation. TLS structures exhibiting a variable degree of maturation are found in solid tumors. They are composed of various immune cell types including dendritic cells and antigen-specific B and T lymphocytes, that together, actively drive the immune response against tumor development and progression. This review highlights the successive steps leading to tumor TLS formation and its association with clinical outcomes. We discuss the role played by tumor-infiltrating B lymphocytes and plasma cells, their prognostic value in solid tumors and immunotherapeutic responses and their potential for future targeting.
Keywords: Tertiary lymphoid structures; b lymphocytes; cancer; immunotherapy; plasma cells.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
