The Active Subunit of the Cytolethal Distending Toxin, CdtB, Derived From Both Haemophilus ducreyi and Campylobacter jejuni Exhibits Potent Phosphatidylinositol-3,4,5-Triphosphate Phosphatase Activity
- PMID: 33854985
- PMCID: PMC8039388
- DOI: 10.3389/fcimb.2021.664221
The Active Subunit of the Cytolethal Distending Toxin, CdtB, Derived From Both Haemophilus ducreyi and Campylobacter jejuni Exhibits Potent Phosphatidylinositol-3,4,5-Triphosphate Phosphatase Activity
Abstract
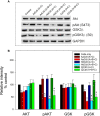
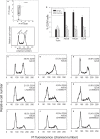
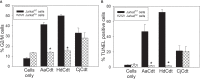
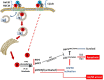
Human lymphocytes exposed to Aggregatibacter actinomycetemcomitans (Aa) cytolethal distending toxin (Cdt) undergo cell cycle arrest and apoptosis. In previous studies, we demonstrated that the active Cdt subunit, CdtB, is a potent phosphatidylinositol (PI) 3,4,5-triphosphate phosphatase. Moreover, AaCdt-treated cells exhibit evidence of PI-3-kinase (PI-3K) signaling blockade characterized by reduced levels of PIP3, pAkt, and pGSK3β. We have also demonstrated that PI-3K blockade is a requisite of AaCdt-induced toxicity in lymphocytes. In this study, we extended our observations to include assessment of Cdts from Haemophilus ducreyi (HdCdt) and Campylobacter jejuni (CjCdt). We now report that the CdtB subunit from HdCdt and CjCdt, similar to that of AaCdt, exhibit potent PIP3 phosphatase activity and that Jurkat cells treated with these Cdts exhibit PI-3K signaling blockade: reduced levels of pAkt and pGSK3β. Since non-phosphorylated GSK3β is the active form of this kinase, we compared Cdts for dependence on GSK3β activity. Two GSK3β inhibitors were employed, LY2090314 and CHIR99021; both inhibitors blocked the ability of Cdts to induce cell cycle arrest. We have previously demonstrated that AaCdt induces increases in the CDK inhibitor, p21CIP1/WAF1, and, further, that this was a requisite for toxin-induced cell death via apoptosis. We now demonstrate that HdCdt and CjCdt also share this requirement. It is also noteworthy that p21CIP1/WAF1 was not involved in the ability of the three Cdts to induce cell cycle arrest. Finally, we demonstrate that, like AaCdt, HdCdt is dependent upon the host cell protein, cellugyrin, for its toxicity (and presumably internalization of CdtB); CjCdt was not dependent upon this protein. The implications of these findings as they relate to Cdt's molecular mode of action are discussed.
Keywords: apoptosis; cell cycle arrest; cytolethal distending toxin; host-parasite interactions; lymphocytes; pathogenesis; toxins.
Copyright © 2021 Huang, Boesze-Battaglia, Walker, Zekavat, Schaefer, Blanke and Shenker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Boesze-Battaglia K., Besack D., McKay T., Zekavat A., Otis L., Jordan-Sciutto K., et al. (2006). Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell Microbiol. 8 (5), 823–836. 10.1111/j.1462-5822.2005.00669.x - DOI - PMC - PubMed
-
- Boesze-Battaglia K., Walker L. P., Zekavat A., Dlakic M., Scuron M. D., Nygren P., et al. (2015). The Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Active Subunit CdtB Contains a Cholesterol Recognition Sequence Required for Toxin Binding and Subunit Internalization. Infect. Immun. 83 (10), 4042–4055. 10.1128/IAI.00788-15 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

