Targeted therapy in eosinophilic chronic obstructive pulmonary disease
- PMID: 33855061
- PMCID: PMC8039900
- DOI: 10.1183/23120541.00437-2020
Targeted therapy in eosinophilic chronic obstructive pulmonary disease
Abstract
Chronic obstructive pulmonary disease (COPD) is a common and preventable airway disease causing significant worldwide mortality and morbidity. Lifetime exposure to tobacco smoking and environmental particles are the two major risk factors. Over recent decades, COPD has become a growing public health problem with an increase in incidence. COPD is defined by airflow limitation due to airway inflammation and small airway remodelling coupled to parenchymal lung destruction. Most patients exhibit neutrophil-predominant airway inflammation combined with an increase in macrophages and CD8+ T-cells. Asthma is a heterogeneous chronic inflammatory airway disease. The most studied subtype is type 2 (T2) high eosinophilic asthma, for which there are an increasing number of biologic agents developed. However, both asthma and COPD are complex and share common pathophysiological mechanisms. They are known as overlapping syndromes as approximately 40% of patients with COPD present an eosinophilic airway inflammation. Several studies suggest a putative role of eosinophilia in lung function decline and COPD exacerbation. Recently, pharmacological agents targeting eosinophilic traits in uncontrolled eosinophilic asthma, especially monoclonal antibodies directed against interleukins (IL-5, IL-4, IL-13) or their receptors, have shown promising results. This review examines data on the rationale for such biological agents and assesses efficacy in T2-endotype COPD patients.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: M. Fieldes has nothing to disclose. Conflict of interest: C. Bourguignon has nothing to disclose. Conflict of interest: S. Assou has nothing to disclose. Conflict of interest: A. Nasri has nothing to disclose. Conflict of interest: A. Fort has nothing to disclose. Conflict of interest: I. Vachier has nothing to disclose. Conflict of interest: J. De Vos has nothing to disclose. Conflict of interest: E. Ahmed has nothing to disclose. Conflict of interest: A. Bourdin reports grants, personal fees, nonfinancial support and other support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Novartis; personal fees and nonfinancial support from Teva; personal fees, nonfinancial support and other support from Regeneron, Chiesi Farmaceuticals and Actelion; personal fees from Gilead; nonfinancial support and other support from Roche; and other support from Nuvaira, all outside the submitted work.
Figures
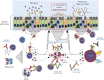
References
-
- IMHE, Institute for Health Metrics and Evaluation (IHME). GBD Compare. Seattle, WA: IHME, University of Washington, 2015. http://www.healthdata.org/data-visualization/gbd-compare Date last accessed: May, 2020; date last updated: May 2020.
-
- GBD 2015 Chronic Respiratory Disease Collaborators . Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. doi:10.1016/S2213-2600(17)30293-X - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
