Subchronic oral toxicity evaluation of gold nanoparticles in male and female mice
- PMID: 33855242
- PMCID: PMC8027780
- DOI: 10.1016/j.heliyon.2021.e06577
Subchronic oral toxicity evaluation of gold nanoparticles in male and female mice
Abstract
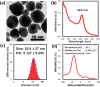
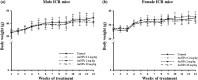
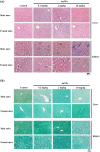
Gold nanoparticles (AuNPs) are biocompatible nanomaterials with potential application in the food industry. The safety of AuNPs oral consumption remains inconclusive, and information on possible long-term toxicity is limited. The current study aimed to evaluate the subchronic oral toxicity of AuNPs in male and female Institute of Cancer Research (ICR) mice. Citrate-coated spherical AuNPs with 53 nm diameters were prepared and orally administered to the mice. No mortality or clinical abnormalities were observed following daily administration of AuNPs at the dosages of 0.2, 2, and 20 mg/kg for 90 days. There was no significant difference in body weight or the relative organs' weights between the control and AuNPs-treated mice. No gross abnormalities or histopathological changes were observed except that the male mice treated with high dose (20 mg/kg AuNPs) showed minor infiltration in the kidneys, and female mice showed a reduced A/G ratio and elevated platelet indices. Overall, the 90-day long-term oral consumption of AuNPs did not cause significant toxicity in mice.
Keywords: Chronic toxicity; Gold; Nanoparticle; Oral; Tissue histopathology.
© 2021 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4- and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice.J Nanobiotechnology. 2018 Nov 1;16(1):86. doi: 10.1186/s12951-018-0415-5. J Nanobiotechnology. 2018. PMID: 30384844 Free PMC article.
-
Investigating the fate and toxicity of green synthesized gold nanoparticles in albino mice.Drug Dev Ind Pharm. 2023 Aug;49(8):508-520. doi: 10.1080/03639045.2023.2243334. Epub 2023 Aug 11. Drug Dev Ind Pharm. 2023. PMID: 37530565
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
Cited by
-
The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models.Int J Mol Sci. 2023 Mar 15;24(6):5634. doi: 10.3390/ijms24065634. Int J Mol Sci. 2023. PMID: 36982705 Free PMC article.
-
Green Synthesis and Antiproliferative Activity of Gold Nanoparticles of a Controlled Size and Shape Obtained Using Shock Wave Extracts from Amphipterygium adstringens.Bioengineering (Basel). 2023 Mar 30;10(4):437. doi: 10.3390/bioengineering10040437. Bioengineering (Basel). 2023. PMID: 37106624 Free PMC article.
-
Gold Nanoparticle-Based Therapy for Muscle Inflammation and Oxidative Stress.J Inflamm Res. 2022 May 31;15:3219-3234. doi: 10.2147/JIR.S327292. eCollection 2022. J Inflamm Res. 2022. PMID: 35668914 Free PMC article. Review.
-
Gold Nanoparticles Down-Regulate Alpha Fetoprotein Expression Induced by Meloxicam Hepatotoxicity in Adult Male Albino Rats: Histological and Immunohistochemical Study.J Microsc Ultrastruct. 2023 Mar 22;13(1):8-15. doi: 10.4103/jmau.jmau_109_22. eCollection 2025 Jan-Mar. J Microsc Ultrastruct. 2023. PMID: 40351743 Free PMC article.
-
Targeted delivery of gold nanoparticles by neural stem cells to glioblastoma for enhanced radiation therapy: a review.AIMS Neurosci. 2022 Jul 8;9(3):303-319. doi: 10.3934/Neuroscience.2022017. eCollection 2022. AIMS Neurosci. 2022. PMID: 36329899 Free PMC article. Review.
References
-
- Chen H., Zhou K., Zhao G. Gold nanoparticles: from synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018;78:83–94.
LinkOut - more resources
Full Text Sources
Other Literature Sources

