Distinct roles and actions of protein disulfide isomerase family enzymes in catalysis of nascent-chain disulfide bond formation
- PMID: 33855279
- PMCID: PMC8024706
- DOI: 10.1016/j.isci.2021.102296
Distinct roles and actions of protein disulfide isomerase family enzymes in catalysis of nascent-chain disulfide bond formation
Abstract
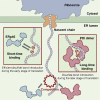
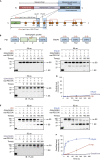
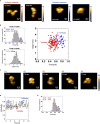
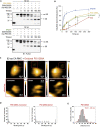
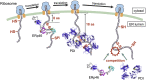
The mammalian endoplasmic reticulum (ER) harbors more than 20 members of the protein disulfide isomerase (PDI) family that act to maintain proteostasis. Herein, we developed an in vitro system for directly monitoring PDI- or ERp46-catalyzed disulfide bond formation in ribosome-associated nascent chains of human serum albumin. The results indicated that ERp46 more efficiently introduced disulfide bonds into nascent chains with a short segment exposed outside the ribosome exit site than PDI. Single-molecule analysis by high-speed atomic force microscopy further revealed that PDI binds nascent chains persistently, forming a stable face-to-face homodimer, whereas ERp46 binds for a shorter time in monomeric form, indicating their different mechanisms for substrate recognition and disulfide bond introduction. Thus, ERp46 serves as a more potent disulfide introducer especially during the early stages of translation, whereas PDI can catalyze disulfide formation when longer nascent chains emerge out from ribosome.
Keywords: Cell Biology; Molecular Biology; Structural Biology.
© 2021 The Author(s).
Conflict of interest statement
We declare that there are no competing interests related to this work.
Figures



Similar articles
-
Radically different thioredoxin domain arrangement of ERp46, an efficient disulfide bond introducer of the mammalian PDI family.Structure. 2014 Mar 4;22(3):431-43. doi: 10.1016/j.str.2013.12.013. Epub 2014 Jan 23. Structure. 2014. PMID: 24462249
-
Regulation of plant ER oxidoreductin 1 (ERO1) activity for efficient oxidative protein folding.J Biol Chem. 2019 Dec 6;294(49):18820-18835. doi: 10.1074/jbc.RA119.010917. Epub 2019 Nov 4. J Biol Chem. 2019. PMID: 31685660 Free PMC article.
-
Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum.Free Radic Biol Med. 2015 Jun;83:314-22. doi: 10.1016/j.freeradbiomed.2015.02.010. Epub 2015 Feb 17. Free Radic Biol Med. 2015. PMID: 25697777 Review.
-
Identification and characterization of GmPDIL7, a soybean ER membrane-bound protein disulfide isomerase family protein.FEBS J. 2017 Feb;284(3):414-428. doi: 10.1111/febs.13984. Epub 2016 Dec 25. FEBS J. 2017. PMID: 27960051
-
Emerging roles of protein disulfide isomerase in cancer.BMB Rep. 2017 Aug;50(8):401-410. doi: 10.5483/bmbrep.2017.50.8.107. BMB Rep. 2017. PMID: 28648146 Free PMC article. Review.
Cited by
-
Polysulfide and persulfide-mediated activation of the PERK-eIF2α-ATF4 pathway increases Sestrin2 expression and reduces methylglyoxal toxicity.Redox Biol. 2025 Feb;79:103450. doi: 10.1016/j.redox.2024.103450. Epub 2024 Dec 5. Redox Biol. 2025. PMID: 39667306 Free PMC article.
-
Distinct role of ERp57 and ERdj5 as a disulfide isomerase and reductase during ER protein folding.J Cell Sci. 2023 Jan 15;136(2):jcs260656. doi: 10.1242/jcs.260656. Epub 2023 Jan 19. J Cell Sci. 2023. PMID: 36655611 Free PMC article.
-
Protein disulphide isomerase A4 as a potential biomarker for coronavirus disease 2019: Correlation with cytokine profiles and disease progression.Virulence. 2025 Dec;16(1):2508815. doi: 10.1080/21505594.2025.2508815. Epub 2025 May 26. Virulence. 2025. PMID: 40391685 Free PMC article.
-
Thioredoxin Domain Containing 5 (TXNDC5): Friend or Foe?Curr Issues Mol Biol. 2024 Apr 4;46(4):3134-3163. doi: 10.3390/cimb46040197. Curr Issues Mol Biol. 2024. PMID: 38666927 Free PMC article. Review.
-
Proteome-wide characterization of PTMs reveals host cell responses to viral infection and identifies putative antiviral drug targets.Front Immunol. 2025 May 30;16:1587106. doi: 10.3389/fimmu.2025.1587106. eCollection 2025. Front Immunol. 2025. PMID: 40519923 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

