Drosophila RASopathy models identify disease subtype differences and biomarkers of drug efficacy
- PMID: 33855281
- PMCID: PMC8026909
- DOI: 10.1016/j.isci.2021.102306
Drosophila RASopathy models identify disease subtype differences and biomarkers of drug efficacy
Abstract
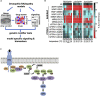
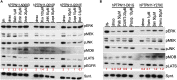
RASopathies represent a family of mostly autosomal dominant diseases that are caused by missense variants in the rat sarcoma viral oncogene/mitogen activated protein kinase (RAS/MAPK) pathway including KRAS, NRAS, BRAF, RAF1, and SHP2. These variants are associated with overlapping but distinct phenotypes that affect the heart, craniofacial, skeletal, lymphatic, and nervous systems. Here, we report an analysis of 13 Drosophila transgenic lines, each expressing a different human RASopathy isoform. Similar to their human counterparts, each Drosophila line displayed common aspects but also important differences including distinct signaling pathways such as the Hippo and SAPK/JNK signaling networks. We identified multiple classes of clinically relevant drugs-including statins and histone deacetylase inhibitors-that improved viability across most RASopathy lines; in contrast, several canonical RAS pathway inhibitors proved less broadly effective. Overall, our study compares and contrasts a large number of RASopathy-associated variants including their therapeutic responses.
Keywords: Biological Sciences; Cell Biology; Molecular Biology; Physiology.
Crown Copyright © 2021.
Conflict of interest statement
B.G. declares royalties from GeneDx, Correlegan, LabCorp, and Prevention Genetics. B.G. is a named inventor on issued patents related to PTPN11, SHOC2, RAF1, and SOS1 mutations in Noonan syndrome. The Icahn School of Medicine at Mount Sinai licensed the patent to several diagnostics companies and has received royalty payments, some of which are distributed to B.G. B.G. and R.C. are principal investigators for a sponsored research agreement from Onconova.
Figures





Similar articles
-
Clinical and mutation profile of pediatric patients with RASopathy-associated hypertrophic cardiomyopathy: results from a Chinese cohort.Orphanet J Rare Dis. 2019 Feb 7;14(1):29. doi: 10.1186/s13023-019-1010-z. Orphanet J Rare Dis. 2019. PMID: 30732632 Free PMC article.
-
RASopathies - what they reveal about RAS/MAPK signaling in skeletal muscle development.Dis Model Mech. 2024 Jun 1;17(6):dmm050609. doi: 10.1242/dmm.050609. Epub 2024 Jun 7. Dis Model Mech. 2024. PMID: 38847227 Free PMC article. Review.
-
Copy number variants including RAS pathway genes-How much RASopathy is in the phenotype?Am J Med Genet A. 2015 Nov;167A(11):2685-90. doi: 10.1002/ajmg.a.37155. Epub 2015 May 14. Am J Med Genet A. 2015. PMID: 25974318
-
NGS testing for cardiomyopathy: Utility of adding RASopathy-associated genes.Hum Mutat. 2018 Jul;39(7):954-958. doi: 10.1002/humu.23535. Epub 2018 May 16. Hum Mutat. 2018. PMID: 29696744 Free PMC article.
-
The RASopathies: from pathogenetics to therapeutics.Dis Model Mech. 2022 Feb 1;15(2):dmm049107. doi: 10.1242/dmm.049107. Epub 2022 Feb 18. Dis Model Mech. 2022. PMID: 35178568 Free PMC article. Review.
Cited by
-
A Targeted, Low-Throughput Compound Screen in a Drosophila Model of Neurofibromatosis Type 1 Identifies Simvastatin and BMS-204352 as Potential Therapies for Autism Spectrum Disorder (ASD).eNeuro. 2023 May 16;10(5):ENEURO.0461-22.2023. doi: 10.1523/ENEURO.0461-22.2023. Print 2023 May. eNeuro. 2023. PMID: 37185294 Free PMC article.
-
Impaired MC3T3-E1 osteoblast differentiation triggered by oncogenic HRAS is rescued by the farnesyltransferase inhibitor Tipifarnib.Sci Rep. 2025 Feb 26;15(1):6832. doi: 10.1038/s41598-025-91592-x. Sci Rep. 2025. PMID: 40000861 Free PMC article.
-
FMRP activity and control of Csw/SHP2 translation regulate MAPK-dependent synaptic transmission.PLoS Biol. 2023 Jan 26;21(1):e3001969. doi: 10.1371/journal.pbio.3001969. eCollection 2023 Jan. PLoS Biol. 2023. PMID: 36701299 Free PMC article.
-
How can same-gene mutations promote both cancer and developmental disorders?Sci Adv. 2022 Jan 14;8(2):eabm2059. doi: 10.1126/sciadv.abm2059. Epub 2022 Jan 14. Sci Adv. 2022. PMID: 35030014 Free PMC article.
-
A Drosophila chemical screen reveals synergistic effect of MEK and DGKα inhibition in Ras-driven cancer.Dis Model Mech. 2023 Mar 1;16(3):dmm049769. doi: 10.1242/dmm.049769. Epub 2023 Apr 3. Dis Model Mech. 2023. PMID: 36861754 Free PMC article.
References
-
- Araki T., Mohi M.G., Ismat F.A., Bronson R.T., Williams I.R., Kutok J.L., Yang W., Pao L.I., Gilliland D.G., Epstein J.A., Neel B.G. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat. Med. 2004;10:849–857. - PubMed
-
- Azad T., Nouri K., Janse van Rensburg H.J., Maritan S.M., Wu L., Hao Y., Montminy T., Yu J., Khanal P., Mulligan L.M., Yang X. A gain-of-functional screen identifies the Hippo pathway as a central mediator of receptor tyrosine kinases during tumorigenesis. Oncogene. 2020;39:334–355. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

