Reward sensitivity and action in Parkinson's disease patients with and without apathy
- PMID: 33855297
- PMCID: PMC8024004
- DOI: 10.1093/braincomms/fcab022
Reward sensitivity and action in Parkinson's disease patients with and without apathy
Abstract
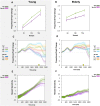
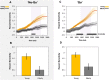
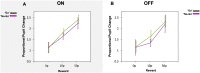
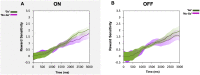
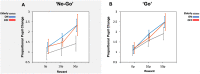
Clinical apathy results in dysfunction of goal directed behaviour, a key component of which is the initiation of action. Previous work has suggested that blunting of reward sensitivity is an important mechanism underlying apathy. However, an additional component might be impoverished initiation of action itself. This study aims to investigate the link between motivation and motor output and its association with apathy and dopamine. An oculomotor task that measures pupillary and saccadic response to monetary incentives was used to assess reward sensitivity, first in 23 young and 18 elderly controls, and then in 22 patients with Parkinson's disease tested ON and OFF dopaminergic medication. To distinguish between pupillary responses to anticipated reward alone versus responses associated with motor preparation, a saccadic 'go/no-go' task was performed. Half of the trials required a saccade to be initiated to receive a reward and in the remaining trials no action was required but reward was still obtained. No significant difference in pupil response was demonstrated between the two conditions in all groups tested, suggesting pupillary responses to rewards are not contingent upon motor preparation in Parkinson's disease. Being ON or OFF dopamine did not influence this response either. Previous work demonstrated associations between apathy and pupillary reward insensitivity in Parkinson's disease. Here we observed this effect only when an action was required to receive a reward, and only in the ON state. These findings suggest that apathy in Parkinson's disease is linked to reduced reward sensitivity and that this is most prominently observed when actions have to be initiated to rewarding goals, with the effect modulated by being ON dopaminergic medication. OFF medication, there was no such strong relationship, and similarly in the 'no-go' conditions, either ON or OFF dopaminergic drugs. The results provide preliminary data which suggest that apathy in Parkinson's disease is associated with a reduction in reward sensitivity and this is most evident when associated with initiation of goal directed actions in the presence of adequate dopamine.
Keywords: Parkinson’s disease; apathy; dopamine; pupillometry; reward sensitivity.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–254. - PubMed
-
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J.. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. - PubMed
-
- Stuss D, Van Reekum R, Murphy K.. Differentiation of states and causes of apathy. In: The Neuropsychology of Emotion. 2000:340–363.
-
- Robert PH, Clairet S, Benoit M, et al.The Apathy Inventory: assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17(12):1099–1105. - PubMed
-
- Starkstein SE, Leentjens AFG.. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79(10):1088–1092. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
