Maternal to fetal transmission of cancer: implications for molecular tumor testing, immune regulation, and pediatric malignancies
- PMID: 33855310
- PMCID: PMC8040028
- DOI: 10.1016/j.medj.2021.02.006
Maternal to fetal transmission of cancer: implications for molecular tumor testing, immune regulation, and pediatric malignancies
Abstract
Molecular tumor testing has transformed the treatment of patients with malignancies and is helping catalyze the development of novel therapeutic strategies. In a recent issue of The New England Journal of Medicine, Arakawa et al. describe two cases of pediatric lung cancer resulting from mother-to-infant transmission, diagnosed remote from delivery.1.
Conflict of interest statement
DECLARATION OF INTERESTS Dr. Eskander receives research funding from Clovis Oncology, Merck, and AstraZenca, as well as consultant and/or speaker fees and/or advisory board from AstraZeneca, GSK/Tesaro, Myriad, Merck, and the GOG Foundation. Dr. Kurzrock receives research funding from Genentech, Merck Serono, Pfizer, Boehringer Ingelheim, TopAlliance, Takeda, Incyte, Debiopharm, Medimmune, Sequenom, Foundation Medicine, Konica Minolta, Grifols, Omniseq, and Guardant, as well as consultant and/or speaker fees and/or advisory board for X-Biotech, Neomed, Pfizer, Actuate Therapeutics, Roche, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics, Inc.; has an equity interest in IDbyDNA and CureMatch Inc; serves on the Board of CureMatch and CureMetrix; and is a co-founder of CureMatch.
Figures
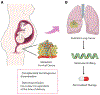
Comment on
-
Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants.N Engl J Med. 2021 Jan 7;384(1):42-50. doi: 10.1056/NEJMoa2030391. N Engl J Med. 2021. PMID: 33406329
References
-
- Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, et al. (2021). Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants.N. Engl. J. Med 384, 42–50. - PubMed
-
- Teksam M, McKinney A, Short J, Casey SO, and Truwit CL (2004). Intracranial metastasis via transplacental (vertical) transmission of maternal small cell lung cancer to fetus: CT and MRI findings. Acta Radiol. 45, 577–579. - PubMed
-
- Herskovic E, Ryan M, Weinstein J, and Wadhwani NR (2014). Maternal to fetal transmission of cervical carcinoma. Pediatr. Radiol 44, 1035–1038. - PubMed
-
- Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, et al. (2017). Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390, 1654–1663. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
