Niclosamide's potential direct targets in ovarian cancer†
- PMID: 33855343
- PMCID: PMC8335355
- DOI: 10.1093/biolre/ioab071
Niclosamide's potential direct targets in ovarian cancer†
Abstract
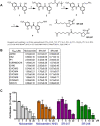
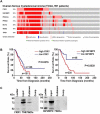
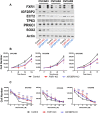
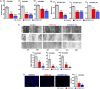
Recent evidence indicates that niclosamide is an anti-cancer compound that is able to inhibit several signaling pathways. Although niclosamide has previously been identified by high-throughput screening platforms as a potential effective compound against several cancer types, no direct binding interactions with distinct biological molecule(s) has been established. The present study identifies key signal transduction mechanisms altered by niclosamide in ovarian cancer. Using affinity purification with a biotin-modified niclosamide derivative and mass spectrometry analysis, several RNA-binding proteins (RBPs) were identified. We chose the two RBPs, FXR1 and IGF2BP2, for further analysis. A significant correlation exists in which high-expression of FXR1 or IGF2BP2 is associated with reduced survival of ovarian cancer patients. Knockdown of FXR1 or IGF2BP2 in ovarian cancer cells resulted in significantly reduced cell viability, adhesion, and migration. Furthermore, FXR1 or IGF2BP2 deficient ovarian cancer cells exhibited reduced response to most doses of niclosamide showing greater cell viability than those with intact RBPs. These results suggest that FXR1 and IGF2BP2 are direct targets of niclosamide and could have critical activities that drive multiple oncogenic pathways in ovarian cancer.
Keywords: FXR1 and IGF2BP2; RNA-binding protein; niclosamide; ovarian cancer.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
A Blockade of IGF Signaling Sensitizes Human Ovarian Cancer Cells to the Anthelmintic Niclosamide-Induced Anti-Proliferative and Anticancer Activities.Cell Physiol Biochem. 2016;39(3):871-88. doi: 10.1159/000447797. Epub 2016 Aug 9. Cell Physiol Biochem. 2016. PMID: 27497986
-
Growth inhibition of ovarian tumor-initiating cells by niclosamide.Mol Cancer Ther. 2012 Aug;11(8):1703-12. doi: 10.1158/1535-7163.MCT-12-0002. Epub 2012 May 10. Mol Cancer Ther. 2012. PMID: 22576131
-
The Anthelmintic Drug Niclosamide Inhibits the Proliferative Activity of Human Osteosarcoma Cells by Targeting Multiple Signal Pathways.Curr Cancer Drug Targets. 2015;15(8):726-38. doi: 10.2174/1568009615666150629132157. Curr Cancer Drug Targets. 2015. PMID: 26118906
-
Multi-targeted therapy of cancer by niclosamide: A new application for an old drug.Cancer Lett. 2014 Jul 10;349(1):8-14. doi: 10.1016/j.canlet.2014.04.003. Epub 2014 Apr 13. Cancer Lett. 2014. PMID: 24732808 Free PMC article. Review.
-
Niclosamide, an old antihelminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells.Chin J Cancer. 2012 Apr;31(4):178-84. doi: 10.5732/cjc.011.10290. Epub 2012 Jan 9. Chin J Cancer. 2012. PMID: 22237038 Free PMC article. Review.
Cited by
-
Drug Repurposing: Research Progress of Niclosamide and Its Derivatives on Antibacterial Activity.Infect Drug Resist. 2024 Oct 21;17:4539-4556. doi: 10.2147/IDR.S490998. eCollection 2024. Infect Drug Resist. 2024. PMID: 39464831 Free PMC article. Review.
-
RNA-binding protein FXR1 drives cMYC translation by recruiting eIF4F complex to the translation start site.Cell Rep. 2021 Nov 2;37(5):109934. doi: 10.1016/j.celrep.2021.109934. Cell Rep. 2021. PMID: 34731628 Free PMC article.
-
YTHDF1 in Tumor Cell Metabolism: An Updated Review.Molecules. 2023 Dec 26;29(1):140. doi: 10.3390/molecules29010140. Molecules. 2023. PMID: 38202722 Free PMC article. Review.
-
Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives.Pharmaceuticals (Basel). 2023 Feb 11;16(2):274. doi: 10.3390/ph16020274. Pharmaceuticals (Basel). 2023. PMID: 37259418 Free PMC article. Review.
-
Insulin-like growth factor 2 mRNA-binding protein 2 is a therapeutic target in ovarian cancer.Exp Biol Med (Maywood). 2023 Dec;248(23):2198-2209. doi: 10.1177/15353702231214268. Epub 2023 Dec 12. Exp Biol Med (Maywood). 2023. PMID: 38084732 Free PMC article.
References
-
- Craig P, Ito A. Intestinal cestodes. Curr Opin Infect Dis 2007; 20:524–532. - PubMed
-
- Merschjohann K, Steverding D. In vitro trypanocidal activity of the anti-helminthic drug niclosamide. Exp Parasitol 2008; 118:637–640. - PubMed
-
- Tanowitz HB, Weiss LM, Wittner M. Diagnosis and treatment of intestinal helminths. I. Common intestinal cestodes. Gastroenterologist 1993; 1:265–273. - PubMed
-
- Fonseca BD, Diering GH, Bidinosti MA, Dalal K, Alain T, Balgi AD, Forestieri R, Nodwell M, Rajadurai CV, Gunaratnam C, Tee AR, Duong F et al. Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem 2012; 287:17530–17545. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

