Baseline Interleukin-6 and -8 predict response and survival in patients with advanced hepatocellular carcinoma treated with sorafenib monotherapy: an exploratory post hoc analysis of the SORAMIC trial
- PMID: 33855585
- PMCID: PMC8800931
- DOI: 10.1007/s00432-021-03627-1
Baseline Interleukin-6 and -8 predict response and survival in patients with advanced hepatocellular carcinoma treated with sorafenib monotherapy: an exploratory post hoc analysis of the SORAMIC trial
Abstract
Purpose: To explore the potential correlation between baseline interleukin (IL) values and overall survival or objective response in patients with hepatocellular carcinoma (HCC) receiving sorafenib.
Methods: A subset of patients with HCC undergoing sorafenib monotherapy within a prospective multicenter phase II trial (SORAMIC, sorafenib treatment alone vs. combined with Y90 radioembolization) underwent baseline IL-6 and IL-8 assessment before treatment initiation. In this exploratory post hoc analysis, the best cut-off points for baseline IL-6 and IL-8 values predicting overall survival (OS) were evaluated, as well as correlation with the objective response.
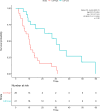
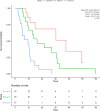
Results: Forty-seven patients (43 male) with a median OS of 13.8 months were analyzed. Cut-off values of 8.58 and 57.9 pg/mL most effectively predicted overall survival for IL-6 and IL-8, respectively. Patients with high IL-6 (HR, 4.1 [1.9-8.9], p < 0.001) and IL-8 (HR, 2.4 [1.2-4.7], p = 0.009) had significantly shorter overall survival than patients with low IL values. Multivariate analysis confirmed IL-6 (HR, 2.99 [1.22-7.3], p = 0.017) and IL-8 (HR, 2.19 [1.02-4.7], p = 0.044) as independent predictors of OS. Baseline IL-6 and IL-8 with respective cut-off values predicted objective response rates according to mRECIST in a subset of 42 patients with follow-up imaging available (IL-6, 46.6% vs. 19.2%, p = 0.007; IL-8, 50.0% vs. 17.4%, p = 0.011).
Conclusion: IL-6 and IL-8 baseline values predicted outcomes of sorafenib-treated patients in this well-characterized prospective cohort of the SORAMIC trial. We suggest that the respective cut-off values might serve for validation in larger cohorts, potentially offering guidance for improved patient selection.
Keywords: Hepatocellular carcinoma; Interleukin; Response; Sorafenib.
© 2021. The Author(s).
Conflict of interest statement
Maciej Pech: Grants: Sirtex, Bayer; Personal fees: Sirtex. Peter Malfertheiner: Grants: Bayer, Sirtex. Jens Ricke: Grants: Sirtex, Bayer; Personal fees: Sirtex, Bayer. Max Seidensticker: Personal fees: Bayer, Sirtex.
Figures



Similar articles
-
miRNome Profiling Analysis Reveals Novel Hepatocellular Carcinoma Diagnostic, Prognostic and Treatment-Related Candidate Biomarkers: Post hoc Analysis of SORAMIC Trial.Dig Dis. 2024;42(4):336-348. doi: 10.1159/000538757. Epub 2024 Apr 24. Dig Dis. 2024. PMID: 38657585
-
High plasma interleukin-6 levels associated with poor prognosis of patients with advanced hepatocellular carcinoma.Jpn J Clin Oncol. 2017 Oct 1;47(10):949-953. doi: 10.1093/jjco/hyx103. Jpn J Clin Oncol. 2017. PMID: 28981732
-
Early tumor shrinkage and response assessment according to mRECIST predict overall survival in hepatocellular carcinoma patients under sorafenib.Cancer Imaging. 2022 Jan 4;22(1):1. doi: 10.1186/s40644-021-00439-x. Cancer Imaging. 2022. PMID: 34983668 Free PMC article.
-
Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma.J Hepatol. 2018 Jul;69(1):60-69. doi: 10.1016/j.jhep.2018.02.008. Epub 2018 Feb 20. J Hepatol. 2018. PMID: 29471013
-
A single center experience of sorafenib in advanced hepatocellular carcinoma patients: evaluation of prognostic factors.Eur J Gastroenterol Hepatol. 2011 Nov;23(12):1233-8. doi: 10.1097/MEG.0b013e32834bd2d0. Eur J Gastroenterol Hepatol. 2011. PMID: 21941188 Review.
Cited by
-
Diagnostic and Prognostic Value of IL-10, FABP2 and LPS Levels in HCC Patients.Medicina (Kaunas). 2023 Dec 17;59(12):2191. doi: 10.3390/medicina59122191. Medicina (Kaunas). 2023. PMID: 38138294 Free PMC article.
-
Suppression of AGTR1 Induces Cellular Senescence in Hepatocellular Carcinoma Through Inactivating ERK Signaling.Front Bioeng Biotechnol. 2022 Jul 13;10:929979. doi: 10.3389/fbioe.2022.929979. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35910032 Free PMC article.
-
Systemic therapies in hepatocellular carcinoma: Existing and emerging biomarkers for treatment response.Front Oncol. 2022 Nov 22;12:1015527. doi: 10.3389/fonc.2022.1015527. eCollection 2022. Front Oncol. 2022. PMID: 36483039 Free PMC article. Review.
-
Time to consider sequencing anti-inflammatory treatments with chemotherapy and immuno-stimulation?Front Immunol. 2022 Dec 22;13:1082142. doi: 10.3389/fimmu.2022.1082142. eCollection 2022. Front Immunol. 2022. PMID: 36618422 Free PMC article. No abstract available.
-
Extracellular Vesicles May Predict Response to Radioembolization and Sorafenib Treatment in Advanced Hepatocellular Carcinoma: An Exploratory Analysis from the SORAMIC Trial.Clin Cancer Res. 2022 Sep 1;28(17):3890-3901. doi: 10.1158/1078-0432.CCR-22-0569. Clin Cancer Res. 2022. PMID: 35763041 Free PMC article. Clinical Trial.
References
-
- Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379(1):54–63 - PMC - PubMed
-
- Bergmann J, Müller M, Baumann N, Reichert M, Heneweer C, Bolik J, Lücke K, Gruber S, Carambia A, Boretius S, Leuschner I, Becker T, Rabe B, Herkel J, Wunderlich FT, Mittrücker HW, Rose-John S, Schmidt-Arras D (2017) IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology 65(1):89–103 - PubMed
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66 - PubMed
-
- Chan SL, Mo FK, Wong CS, Chan CM, Leung LK, Hui EP, Ma BB, Chan AT, Mok TS, Yeo W (2012) A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 118(16):3984–3992 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

