Mannan-BAM, TLR ligands, and anti-CD40 immunotherapy in established murine pancreatic adenocarcinoma: understanding therapeutic potentials and limitations
- PMID: 33855601
- PMCID: PMC9927628
- DOI: 10.1007/s00262-021-02920-9
Mannan-BAM, TLR ligands, and anti-CD40 immunotherapy in established murine pancreatic adenocarcinoma: understanding therapeutic potentials and limitations
Abstract
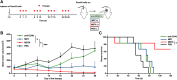
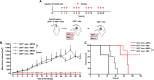
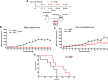
Pancreatic adenocarcinoma is one of the leading causes of cancer-related deaths, and its therapy remains a challenge. Our proposed therapeutic approach is based on the intratumoral injections of mannan-BAM, toll-like receptor ligands, and anti-CD40 antibody (thus termed MBTA therapy), and has shown promising results in the elimination of subcutaneous murine melanoma, pheochromocytoma, colon carcinoma, and smaller pancreatic adenocarcinoma (Panc02). Here, we tested the short- and long-term effects of MBTA therapy in established subcutaneous Panc02 tumors two times larger than in previous study and bilateral Panc02 models as well as the roles of CD4+ and CD8+ T lymphocytes in this therapy. The MBTA therapy resulted in eradication of 67% of Panc02 tumors with the development of long-term memory as evidenced by the rejection of Panc02 cells after subcutaneous and intracranial transplantations. The initial Panc02 tumor elimination is not dependent on the presence of CD4+ T lymphocytes, although these cells seem to be important in long-term survival and resistance against tumor retransplantation. The resistance was revealed to be antigen-specific due to its inability to reject B16-F10 melanoma cells. In the bilateral Panc02 model, MBTA therapy manifested a lower therapeutic response. Despite numerous combinations of MBTA therapy with other therapeutic approaches, our results show that only simultaneous application of MBTA therapy into both tumors has potential for the treatment of the bilateral Panc02 model.
Keywords: Cancer immunotherapy; Checkpoint inhibitors; Mannan; Metastases; Pancreatic adenocarcinoma; TLR ligands.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
The combination of immunotherapy and a glutamine metabolism inhibitor represents an effective therapeutic strategy for advanced and metastatic murine pancreatic adenocarcinoma.Int Immunopharmacol. 2023 May;118:110150. doi: 10.1016/j.intimp.2023.110150. Epub 2023 Apr 6. Int Immunopharmacol. 2023. PMID: 37030115 Free PMC article.
-
Effective cancer immunotherapy based on combination of TLR agonists with stimulation of phagocytosis.Int Immunopharmacol. 2018 Jun;59:86-96. doi: 10.1016/j.intimp.2018.03.038. Epub 2018 Apr 7. Int Immunopharmacol. 2018. PMID: 29635103
-
Neoadjuvant intratumoral MBT(A) immunotherapy prevents distant metastases and recurrence in murine models.Cancer Lett. 2025 Mar 1;612:217464. doi: 10.1016/j.canlet.2025.217464. Epub 2025 Jan 12. Cancer Lett. 2025. PMID: 39809356
-
Mannan-BAM, TLR Ligands, Anti-CD40 Antibody (MBTA) Vaccine Immunotherapy: A Review of Current Evidence and Applications in Glioblastoma.Int J Mol Sci. 2021 Mar 26;22(7):3455. doi: 10.3390/ijms22073455. Int J Mol Sci. 2021. PMID: 33810617 Free PMC article. Review.
-
Role of B cells in intratumoral MBTA immunotherapy of murine pheochromocytoma model.Best Pract Res Clin Endocrinol Metab. 2025 Jan;39(1):101941. doi: 10.1016/j.beem.2024.101941. Epub 2024 Sep 11. Best Pract Res Clin Endocrinol Metab. 2025. PMID: 39278811 Review.
Cited by
-
The combination of immunotherapy and a glutamine metabolism inhibitor represents an effective therapeutic strategy for advanced and metastatic murine pancreatic adenocarcinoma.Int Immunopharmacol. 2023 May;118:110150. doi: 10.1016/j.intimp.2023.110150. Epub 2023 Apr 6. Int Immunopharmacol. 2023. PMID: 37030115 Free PMC article.
-
rWTC-MBTA: autologous vaccine prevents metastases via antitumor immune responses.J Exp Clin Cancer Res. 2023 Jul 12;42(1):163. doi: 10.1186/s13046-023-02744-8. J Exp Clin Cancer Res. 2023. PMID: 37434263 Free PMC article.
-
Identification of Immune Cell Infiltration in Murine Pheochromocytoma during Combined Mannan-BAM, TLR Ligand, and Anti-CD40 Antibody-Based Immunotherapy.Cancers (Basel). 2021 Aug 5;13(16):3942. doi: 10.3390/cancers13163942. Cancers (Basel). 2021. PMID: 34439097 Free PMC article.
-
Injecting hope: the potential of intratumoral immunotherapy for locally advanced and metastatic cancer.Front Immunol. 2025 Jan 9;15:1479483. doi: 10.3389/fimmu.2024.1479483. eCollection 2024. Front Immunol. 2025. PMID: 39850897 Free PMC article. Review.
-
Intratumoral immunotherapy of murine pheochromocytoma shows no age-dependent differences in its efficacy.Front Endocrinol (Lausanne). 2023 May 8;14:1030412. doi: 10.3389/fendo.2023.1030412. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37342258 Free PMC article.
References
-
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. - DOI - PMC - PubMed
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

