Heparin dosage, level, and resistance in SARS-CoV2 infected patients in intensive care unit
- PMID: 33855802
- PMCID: PMC8251410
- DOI: 10.1111/ijlh.13543
Heparin dosage, level, and resistance in SARS-CoV2 infected patients in intensive care unit
Abstract
Introduction: Patients with COVID-19 frequently exhibit a hypercoagulable state with high thrombotic risk, particularly those admitted to intensive care units (ICU). Thromboprophylaxis is mandatory in these patients; nevertheless, thrombosis still occurs in many cases. Thus, the problem of assessing an adequate level of anticoagulation in ICU patients becomes evident during the COVID-19 pandemic. The aim of this study was to evaluate the heparin resistance and the efficacy of heparin monitoring using an anti-Xa activity assay.
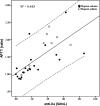
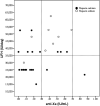
Methods: Thirty-seven heparin-treated patients admitted to ICU for SARS-CoV-2 pneumonia were retrospectively studied for antifactor Xa activity (anti-Xa), activated partial thromboplastin time (APTT), Antithrombin, Fibrinogen, D-Dimer, Factor VIII, von Willebrand Factor, and the total daily amount of heparin administered. The correlation between APTT and anti-Xa was evaluated for unfractionated heparins (UFH). The correlations between the daily dose of UFH or the dosage expressed as IU/kg b.w. for low molecular weight heparin (LMWH) and anti-Xa were also evaluated.
Results: Twenty-one patients received calcium heparin, 8 sodium heparin, and 8 LMWH. A moderate correlation was found between APTT and anti-Xa for UFH. APTT did not correlate with coagulation parameters. 62% of UFH and 75% of LMWH treated patients were under the therapeutic range. About 75% of patients could be considered resistant to heparin.
Conclusions: SARS-COV2 pneumonia patients in ICU have frequently heparin resistance. Anti-Xa seems a more reliable method to monitor heparin treatment than APTT in acute patients, also because the assay is insensitive to the increased levels of fibrinogen, FVIII, and LAC that are common during the COVID-19 inflammatory state.
Keywords: COVID-19; activated partial thromboplastin time; anti-Xa; anticoagulant monitoring; critical care; heparin resistance.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
Dr Chiara Novelli has received lecture fees and consultancy fees from Instrumentation Laboratory‐Werfen, outside the scope of the submitted work. The other authors have no competing interests.
Figures
Comment in
-
Resistance or pitfall in heparin monitoring: An ongoing issue in COVID-19 anticoagulation.Int J Lab Hematol. 2022 Aug;44(4):e135-e137. doi: 10.1111/ijlh.13778. Epub 2021 Dec 16. Int J Lab Hematol. 2022. PMID: 34914184 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

